| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 05:56:33 UTC |
|---|
| Update Date | 2016-11-09 01:21:21 UTC |
|---|
| Accession Number | CHEM035631 |
|---|
| Identification |
|---|
| Common Name | 4-(2-Amino-3-hydroxyphenyl)-2,4-dioxobutanoic acid |
|---|
| Class | Small Molecule |
|---|
| Description | A dioxo monocarboxylic acid that is benzene in which the hydrogens at position 1, 2 and 3 are replaced by 3-carboxy-3-oxopropanoyl, amino and hydroxy groups, respectively. It is a by-product of tryptophan metabolism. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
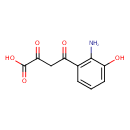
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-Amino-3-hydroxy-alpha,gamma-dioxobenzenebutanoic acid | ChEBI | | 2-Amino-3-hydroxy-a,g-dioxobenzenebutanoate | Generator | | 2-Amino-3-hydroxy-a,g-dioxobenzenebutanoic acid | Generator | | 2-Amino-3-hydroxy-alpha,gamma-dioxobenzenebutanoate | Generator | | 2-Amino-3-hydroxy-α,γ-dioxobenzenebutanoate | Generator | | 2-Amino-3-hydroxy-α,γ-dioxobenzenebutanoic acid | Generator | | 4-(2-Amino-3-hydroxyphenyl)-2,4-dioxobutanoate | Generator |
|
|---|
| Chemical Formula | C10H9NO5 |
|---|
| Average Molecular Mass | 223.182 g/mol |
|---|
| Monoisotopic Mass | 223.048 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 4-(2-amino-3-hydroxyphenyl)-2,4-dioxobutanoic acid |
|---|
| Traditional Name | 4-(2-amino-3-hydroxyphenyl)-2,4-dioxobutanoic acid |
|---|
| SMILES | NC1=C(C=CC=C1O)C(=O)CC(=O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C10H9NO5/c11-9-5(2-1-3-6(9)12)7(13)4-8(14)10(15)16/h1-3,12H,4,11H2,(H,15,16) |
|---|
| InChI Key | YCJNYHCCOXVYAF-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as alkyl-phenylketones. These are aromatic compounds containing a ketone substituted by one alkyl group, and a phenyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbonyl compounds |
|---|
| Direct Parent | Alkyl-phenylketones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alkyl-phenylketone
- Butyrophenone
- Gamma-keto acid
- O-aminophenol
- Aryl alkyl ketone
- Aniline or substituted anilines
- Aminophenol
- Benzoyl
- Phenol
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- 1,3-diketone
- Keto acid
- Benzenoid
- 1,3-dicarbonyl compound
- Alpha-keto acid
- Monocyclic benzene moiety
- Vinylogous amide
- Alpha-hydroxy ketone
- Amino acid or derivatives
- Amino acid
- Carboxylic acid
- Carboxylic acid derivative
- Monocarboxylic acid or derivatives
- Primary amine
- Hydrocarbon derivative
- Organopnictogen compound
- Organic oxide
- Organic nitrogen compound
- Amine
- Organonitrogen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0553-7910000000-894d2b28fa25786b8cf4 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-00dl-9384000000-d85d64127c1a306053b0 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0abi-0690000000-607a25e04b41184c2e11 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0910000000-f6e7ca1f649c7d85f1c8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052u-9800000000-9abd1533cae37b5a1e31 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-2290000000-db5113faa78a8f868817 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0fi0-2940000000-5c30994df2e0c448d78f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-4900000000-5c2c2b7f0e47291f345d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-056r-0900000000-98462e5bccca7cad48a3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-2900000000-fd75faf4a2b73326af8e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-4900000000-564c7472238de91e0b05 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0930000000-b4c5ebc1b69491058158 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-06ri-2900000000-6b672118eb0609fb3d7e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-053r-9300000000-ee7e4c997d23d017bcb6 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0004083 |
|---|
| FooDB ID | FDB023305 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | 46189 |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 389612 |
|---|
| ChEBI ID | 27593 |
|---|
| PubChem Compound ID | 440741 |
|---|
| Kegg Compound ID | C05645 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|