| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 05:53:40 UTC |
|---|
| Update Date | 2016-11-09 01:21:20 UTC |
|---|
| Accession Number | CHEM035571 |
|---|
| Identification |
|---|
| Common Name | 6,7-Dimethyl-8-(1-D-ribityl)lumazine |
|---|
| Class | Small Molecule |
|---|
| Description | 6,7-Dimethyl-8-(1-D-ribityl)lumazine belongs to the class of organic compounds known as pteridines and derivatives. These are polycyclic aromatic compounds containing a pteridine moiety, which consists of a pyrimidine fused to a pyrazine ring to form pyrimido(4,5-b)pyrazine. 6,7-Dimethyl-8-(1-D-ribityl)lumazine is possibly soluble (in water) and an extremely weak basic (essentially neutral) compound (based on its pKa). 6,7-Dimethyl-8-(1-D-ribityl)lumazine exists in all living organisms, ranging from bacteria to humans. In cattle, 6,7-dimethyl-8-(1-D-ribityl)lumazine is involved in the metabolic pathway called the riboflavin metabolism pathway. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
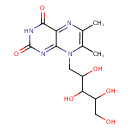
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-Deoxy-1-(3,4-dihydro-6,7-dimethyl-2,4-dioxo-8(2H)-pteridinyl)-ribitol | HMDB | | 1-Deoxy-1-[6,7-dimethyl-2,4-dioxo-3,4-dihydropteridin-8(2H)-yl]-D-ribitol | HMDB | | 6,7-Dimethyl-8-(1'-D-ribityl)lumazine | HMDB | | 6,7-Dimethyl-8-ribityllumazine | HMDB | | 6,7-Dimethyl-8-[(2S,3S,4R)-2,3,4,5-tetrahydroxypentyl]pteridine-2,4-dione | HMDB | | DMDRL | HMDB |
|
|---|
| Chemical Formula | C13H18N4O6 |
|---|
| Average Molecular Mass | 326.305 g/mol |
|---|
| Monoisotopic Mass | 326.123 g/mol |
|---|
| CAS Registry Number | 5118-16-1 |
|---|
| IUPAC Name | 6,7-dimethyl-8-(2,3,4,5-tetrahydroxypentyl)-2,3,4,8-tetrahydropteridine-2,4-dione |
|---|
| Traditional Name | 6,7-dimethyl-8-(2,3,4,5-tetrahydroxypentyl)-3H-pteridine-2,4-dione |
|---|
| SMILES | CC1=C(C)N(CC(O)C(O)C(O)CO)C2=NC(=O)NC(=O)C2=N1 |
|---|
| InChI Identifier | InChI=1S/C13H18N4O6/c1-5-6(2)17(3-7(19)10(21)8(20)4-18)11-9(14-5)12(22)16-13(23)15-11/h7-8,10,18-21H,3-4H2,1-2H3,(H,16,22,23) |
|---|
| InChI Key | SXDXRJZUAJBNFL-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pteridines and derivatives. These are polycyclic aromatic compounds containing a pteridine moiety, which consists of a pyrimidine fused to a pyrazine ring to form pyrimido(4,5-b)pyrazine. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pteridines and derivatives |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Pteridines and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pteridine
- Pyrimidone
- Pyrazine
- Pyrimidine
- Heteroaromatic compound
- Vinylogous amide
- Lactam
- Secondary alcohol
- Polyol
- Azacycle
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Primary alcohol
- Alcohol
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Hydrocarbon derivative
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0bt9-9031000000-9c630ca6d5cf3abd4fa7 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-0k92-2122193000-4fdebb2ae42933ee8a1e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a6r-1059000000-bb2238e34adbb8c00787 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0bt9-4191000000-b3b1e68b02bd73ae782a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-056r-4940000000-f6a2f6ffebaa7bc97464 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0089-3090000000-c6c55b4bb2cd8266bc7e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-9100000000-102fea61448a18c76296 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9200000000-d5a60b46dbd297e60a53 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0003826 |
|---|
| FooDB ID | FDB023230 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | DIMETHYL-D-RIBITYL-LUMAZINE |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 652 |
|---|
| ChEBI ID | 17601 |
|---|
| PubChem Compound ID | 672 |
|---|
| Kegg Compound ID | C04332 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | ECMDB03826 |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|