| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 05:52:35 UTC |
|---|
| Update Date | 2016-11-09 01:21:20 UTC |
|---|
| Accession Number | CHEM035549 |
|---|
| Identification |
|---|
| Common Name | VPGPR Enterostatin |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
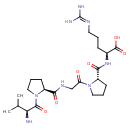
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-[1-[N-(1-L-Valyl-L-prolyl)glycyl]-L-prolyl]-L-arginine | HMDB | | L-Valyl-L-prolylglycyl-L-prolyl-L-arginine | HMDB | | (2S)-2-({[(2S)-1-[2-({[(2S)-1-[(2S)-2-amino-3-methylbutanoyl]pyrrolidin-2-yl](hydroxy)methylidene}amino)acetyl]pyrrolidin-2-yl](hydroxy)methylidene}amino)-5-carbamimidamidopentanoate | Generator, HMDB |
|
|---|
| Chemical Formula | C23H40N8O6 |
|---|
| Average Molecular Mass | 524.614 g/mol |
|---|
| Monoisotopic Mass | 524.307 g/mol |
|---|
| CAS Registry Number | 144964-56-7 |
|---|
| IUPAC Name | (2S)-2-{[(2S)-1-(2-{[(2S)-1-[(2S)-2-amino-3-methylbutanoyl]pyrrolidin-2-yl]formamido}acetyl)pyrrolidin-2-yl]formamido}-5-[(diaminomethylidene)amino]pentanoic acid |
|---|
| Traditional Name | (2S)-2-{[(2S)-1-(2-{[(2S)-1-[(2S)-2-amino-3-methylbutanoyl]pyrrolidin-2-yl]formamido}acetyl)pyrrolidin-2-yl]formamido}-5-[(diaminomethylidene)amino]pentanoic acid |
|---|
| SMILES | CC(C)[C@H](N)C(=O)N1CCC[C@H]1C(=O)NCC(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCN=C(N)N)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C23H40N8O6/c1-13(2)18(24)21(35)31-11-5-7-15(31)19(33)28-12-17(32)30-10-4-8-16(30)20(34)29-14(22(36)37)6-3-9-27-23(25)26/h13-16,18H,3-12,24H2,1-2H3,(H,28,33)(H,29,34)(H,36,37)(H4,25,26,27)/t14-,15-,16-,18-/m0/s1 |
|---|
| InChI Key | QVZKZDOWTVQTPL-OVWQWFNUSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as oligopeptides. These are organic compounds containing a sequence of between three and ten alpha-amino acids joined by peptide bonds. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Oligopeptides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-oligopeptide
- N-acyl-alpha-amino acid
- N-acyl-alpha amino acid or derivatives
- Valine or derivatives
- Proline or derivatives
- N-acyl-l-alpha-amino acid
- Alpha-amino acid amide
- Alpha-amino acid or derivatives
- N-acylpyrrolidine
- Pyrrolidine carboxylic acid or derivatives
- Pyrrolidine-2-carboxamide
- Branched fatty acid
- Heterocyclic fatty acid
- Fatty acyl
- Fatty acid
- Pyrrolidine
- Tertiary carboxylic acid amide
- Amino acid or derivatives
- Carboxamide group
- Guanidine
- Amino acid
- Secondary carboxylic acid amide
- Carboximidamide
- Organoheterocyclic compound
- Propargyl-type 1,3-dipolar organic compound
- Azacycle
- Organic 1,3-dipolar compound
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Primary amine
- Primary aliphatic amine
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Carbonyl group
- Hydrocarbon derivative
- Amine
- Organonitrogen compound
- Organooxygen compound
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0fi0-9331300000-d2a1d2e5ebbba667634a | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00di-9321020000-48a69e256a3399d3c208 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-6864490000-90c6f290f6c618dbd5e8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00fr-9531000000-e07d244d22eecaeb5ac3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-9310000000-a6c8363faab096a3f22c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-1000920000-8fcb3a8178d7cf59bea6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-053r-8434910000-b6478147066844a932f4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0abc-9310000000-c9f3617f8f1896db08eb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-1000490000-fbd0aec065f15bfea07d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-05fr-1101930000-078c1f95830b442c54b2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9421100000-d3f11d79314fc69b26cb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0001190000-15f8ae07ad55b89cc515 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05di-4332490000-ef2cb92d58eb9a213b53 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-6490000000-ea74bb27e91e11b6dcbf | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0003577 |
|---|
| FooDB ID | FDB023200 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 8090424 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 9914775 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Prasad C, Imamura M, Debata C, Svec F, Sumar N, Hermon-Taylor J: Hyperenterostatinemia in premenopausal obese women. J Clin Endocrinol Metab. 1999 Mar;84(3):937-41. | | 2. Ashmarin IP, Karazeeva EP, Lyapina LA, Samonina GE: The simplest proline-containing peptides PG, GP, PGP, and GPGG: regulatory activity and possible sources of biosynthesis. Biochemistry (Mosc). 1998 Feb;63(2):119-24. | | 3. Rytter E, Erlanson-Albertsson C, Lindahl L, Lundquist I, Viberg U, Akesson B, Oste R: Changes in plasma insulin, enterostatin, and lipoprotein levels during an energy-restricted dietary regimen including a new oat-based liquid food. Ann Nutr Metab. 1996;40(4):212-20. |
|
|---|