| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 05:52:09 UTC |
|---|
| Update Date | 2016-11-09 01:21:20 UTC |
|---|
| Accession Number | CHEM035540 |
|---|
| Identification |
|---|
| Common Name | Inositol 1,3,4,5,6-pentakisphosphate |
|---|
| Class | Small Molecule |
|---|
| Description | Inositol 1,3,4,5,6-pentakisphosphate, also known as inositol 1,3,4,5,6-pentakisphosphate, belongs to the class of organic compounds known as inositol phosphates. Inositol phosphates are compounds containing a phosphate group attached to an inositol (or cyclohexanehexol) moiety. Inositol 1,3,4,5,6-pentakisphosphate is possibly soluble (in water) and an extremely strong acidic compound (based on its pKa). Inositol 1,3,4,5,6-pentakisphosphate exists in all living species, ranging from bacteria to humans. Inositol 1,3,4,5,6-pentakisphosphate participates in a number of enzymatic reactions, within cattle. In particular, Inositol 1,3,4,5,6-pentakisphosphate can be biosynthesized from 1D-myo-inositol 1,4,5,6-tetrakisphosphate; which is mediated by the enzyme inositol polyphosphate multikinase. In addition, Inositol 1,3,4,5,6-pentakisphosphate can be converted into D-myo-inositol 3,4,5,6-tetrakisphosphate through the action of the enzyme inositol-tetrakisphosphate 1-kinase. In cattle, inositol 1,3,4,5,6-pentakisphosphate is involved in the metabolic pathway called the inositol metabolism pathway. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
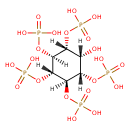
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1D-myo-Inositol 1,3,4,5,6-pentakisphosphate | ChEBI | | D-myo-Inositol 1,3,4,5,6-pentakisphosphate | ChEBI | | 1D-myo-Inositol 1,3,4,5,6-pentakisphosphoric acid | Generator | | Inositol 1,3,4,5,6-pentakisphosphoric acid | Generator | | D-myo-Inositol 1,3,4,5,6-pentakisphosphoric acid | Generator | | Inositol 1,3,4,5,6-pentaphosphate | HMDB | | Inositol pentaphosphate | HMDB | | myo-Inositol 1,3,4,5,6-pentakis(phosphate) | HMDB | | myo-Inositol 1,3,4,5,6-pentaphosphate | HMDB | | myo-Inositol pentakisphosphate | HMDB | | I(1,3,4,5,6)P5 | HMDB | | Inositol 1,3,4,5,6-pentakisphosphate | HMDB | | Ins(1,3,4,5,6)P5 | HMDB | | myo-inositol 1,3,4,5,6-pentakisphosphate | HMDB |
|
|---|
| Chemical Formula | C6H17O21P5 |
|---|
| Average Molecular Mass | 580.055 g/mol |
|---|
| Monoisotopic Mass | 579.895 g/mol |
|---|
| CAS Registry Number | 20298-95-7 |
|---|
| IUPAC Name | {[(1R,2S,3r,4R,5S,6r)-3-hydroxy-2,4,5,6-tetrakis(phosphonooxy)cyclohexyl]oxy}phosphonic acid |
|---|
| Traditional Name | [(1R,2S,3r,4R,5S,6r)-3-hydroxy-2,4,5,6-tetrakis(phosphonooxy)cyclohexyl]oxyphosphonic acid |
|---|
| SMILES | O[C@@H]1[C@H](OP(O)(O)=O)[C@@H](OP(O)(O)=O)[C@H](OP(O)(O)=O)[C@@H](OP(O)(O)=O)[C@@H]1OP(O)(O)=O |
|---|
| InChI Identifier | InChI=1S/C6H17O21P5/c7-1-2(23-28(8,9)10)4(25-30(14,15)16)6(27-32(20,21)22)5(26-31(17,18)19)3(1)24-29(11,12)13/h1-7H,(H2,8,9,10)(H2,11,12,13)(H2,14,15,16)(H2,17,18,19)(H2,20,21,22)/t1-,2+,3-,4-,5+,6+ |
|---|
| InChI Key | CTPQAXVNYGZUAJ-KXXVROSKSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as inositol phosphates. Inositol phosphates are compounds containing a phosphate group attached to an inositol (or cyclohexanehexol) moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Alcohols and polyols |
|---|
| Direct Parent | Inositol phosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Inositol phosphate
- Monoalkyl phosphate
- Cyclohexanol
- Alkyl phosphate
- Phosphoric acid ester
- Organic phosphoric acid derivative
- Secondary alcohol
- Organic oxide
- Hydrocarbon derivative
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-000t-9022820000-0b347c8feac8081572f2 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0udj-8421291000-e7a6038279ed0bab458b | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS ("myo-Inositol 1,3,4,5,6-pentakisphosphate,1TMS,#1" TMS) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_4) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_5) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_6) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_4) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_5) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_6) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - n/a 40V, negative | splash10-0udi-1000900000-189387da7b3691739738 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - n/a 40V, negative | splash10-001i-0009000000-d8e4bd96982581664cb8 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - n/a 40V, negative | splash10-06si-0976000000-4348a6ca785e225be82b | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - n/a 40V, negative | splash10-0udi-0000900000-73573d716178b0ffb8dd | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - n/a 40V, negative | splash10-0udi-0109000000-21271275bbff7f497e41 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - n/a 40V, negative | splash10-08gi-0977000000-4950d48887941a016c30 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - n/a 40V, negative | splash10-004i-9000000000-46d4cee1b5ac630ba9b8 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - n/a 40V, negative | splash10-052r-0900000000-4df79b7aad7183429c3e | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - n/a 40V, negative | splash10-0a4i-0941000000-ebf11ba55edea9d0d03c | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - n/a 40V, negative | splash10-0udi-0109000000-4701557ed0d5f78bceee | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - n/a 40V, negative | splash10-0a4i-0970000000-64206d567dce176ff875 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - n/a 40V, negative | splash10-001i-0009200000-ddb1fa1c37d0ac5a6c75 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - n/a 40V, negative | splash10-06ri-0977000000-778f76090718afa48252 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 23V, negative | splash10-004i-0000090000-09b887f4588457992521 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 26V, negative | splash10-004i-0000290000-99907810b58601057eb2 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 30V, negative | splash10-0059-0000890000-1db30e238cc14e69ef7c | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 32V, negative | splash10-003r-0000940000-cdf319ca2ef7756b6fa2 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 39V, negative | splash10-001i-0102900000-2a426dc0f4578da92fc2 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 49V, negative | splash10-0kcr-0816900000-c79cbf49bffaaf498cbe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-2000590000-a78e46f1069068bb0c1a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01q9-1000390000-aeca364d94e2aaa6c4d6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-2019200000-69fb19c69da99cd9f2d2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-4000190000-e7d2cc4d7c8bcd94a4e4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-9000220000-c245a52aad69af0e69ae | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9000000000-2809ce202dff18cc242c | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0003529 |
|---|
| FooDB ID | FDB023187 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | 37275 |
|---|
| BioCyc ID | CPD-1107 |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 10333900 |
|---|
| ChEBI ID | 16322 |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | C01284 |
|---|
| YMDB ID | YMDB00946 |
|---|
| ECMDB ID | ECMDB21516 |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Riley AM, Trusselle M, Kuad P, Borkovec M, Cho J, Choi JH, Qian X, Shears SB, Spiess B, Potter BV: scyllo-inositol pentakisphosphate as an analogue of myo-inositol 1,3,4,5,6-pentakisphosphate: chemical synthesis, physicochemistry and biological applications. Chembiochem. 2006 Jul;7(7):1114-22. | | 2. Barker CJ, Wright J, Hughes PJ, Kirk CJ, Michell RH: Complex changes in cellular inositol phosphate complement accompany transit through the cell cycle. Biochem J. 2004 Jun 1;380(Pt 2):465-73. |
|
|---|