| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 05:50:57 UTC |
|---|
| Update Date | 2016-11-09 01:21:19 UTC |
|---|
| Accession Number | CHEM035526 |
|---|
| Identification |
|---|
| Common Name | Formyl-CoA |
|---|
| Class | Small Molecule |
|---|
| Description | Formyl-CoA, also known as formyl-coa, belongs to the class of organic compounds known as coenzyme a and derivatives. These are derivative of vitamin B5 containing a 4'-phosphopantetheine moiety attached to a diphospho-adenosine. Formyl-CoA is possibly soluble (in water) and a strong basic compound (based on its pKa). In cattle, formyl-CoA is involved in a couple of metabolic pathways, which include the phytanic Acid peroxisomal oxidation pathway and the oxidation OF branched-chain fatty acids pathway. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
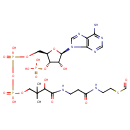
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Formyl coenzyme A | HMDB | | Formyl-coenzyme A | HMDB |
|
|---|
| Chemical Formula | C22H36N7O17P3S |
|---|
| Average Molecular Mass | 795.544 g/mol |
|---|
| Monoisotopic Mass | 795.110 g/mol |
|---|
| CAS Registry Number | 13131-49-2 |
|---|
| IUPAC Name | {[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-2-[({[({3-[(2-{[2-(formylsulfanyl)ethyl]carbamoyl}ethyl)carbamoyl]-3-hydroxy-2,2-dimethylpropoxy}(hydroxy)phosphoryl)oxy](hydroxy)phosphoryl}oxy)methyl]-4-hydroxyoxolan-3-yl]oxy}phosphonic acid |
|---|
| Traditional Name | [(2R,3S,4R,5R)-5-(6-aminopurin-9-yl)-2-{[({3-[(2-{[2-(formylsulfanyl)ethyl]carbamoyl}ethyl)carbamoyl]-3-hydroxy-2,2-dimethylpropoxy(hydroxy)phosphoryl}oxy(hydroxy)phosphoryl)oxy]methyl}-4-hydroxyoxolan-3-yl]oxyphosphonic acid |
|---|
| SMILES | CC(C)(COP(O)(=O)OP(O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP(O)(O)=O)N1C=NC2=C1N=CN=C2N)C(O)C(=O)NCCC(=O)NCCSC=O |
|---|
| InChI Identifier | InChI=1S/C22H36N7O17P3S/c1-22(2,17(33)20(34)25-4-3-13(31)24-5-6-50-11-30)8-43-49(40,41)46-48(38,39)42-7-12-16(45-47(35,36)37)15(32)21(44-12)29-10-28-14-18(23)26-9-27-19(14)29/h9-12,15-17,21,32-33H,3-8H2,1-2H3,(H,24,31)(H,25,34)(H,38,39)(H,40,41)(H2,23,26,27)(H2,35,36,37)/t12-,15-,16-,17?,21-/m1/s1 |
|---|
| InChI Key | SXMOKYXNAPLNCW-BWGWEBPHSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as androgens and derivatives. These are 3-hydroxylated C19 steroid hormones. They are known to favor the development of masculine characteristics. They also show profound effects on scalp and body hair in humans. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Androstane steroids |
|---|
| Direct Parent | Androgens and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Androgen-skeleton
- Oxosteroid
- 17-oxosteroid
- 3-oxosteroid
- 3-oxo-delta-1,4-steroid
- Delta-1,4-steroid
- Cyclic ketone
- Ketone
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0002-9243455200-7fe33bf6d4a771fe5806 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_4) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_5) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_6) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_7) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_8) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-1910000300-55d9e03540fe47686395 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-1930000000-f7d8ae48e314d038f7b2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-2910000000-b160c588de2d041e4b80 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-003r-5820231900-1838b37d7ea4f52f4b46 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-003r-3910100100-933e5170643e8bcc224c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-057i-5900000000-7b6b8f24e737e82fc12e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0000000900-9964bca7fa7ce013e511 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0032-2100003900-ec87a8b97c33e09176bb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-002b-9002417200-07e062501318a903fb23 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0300000900-581000df4cf64f4f5393 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-1910000200-eab4676c05a4b9c71eae | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-1490000000-0296bea622f0a9d9f65c | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0003419 |
|---|
| FooDB ID | FDB023169 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | 36030 |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Acyl-CoA |
|---|
| Chemspider ID | 388444 |
|---|
| ChEBI ID | 15522 |
|---|
| PubChem Compound ID | 439313 |
|---|
| Kegg Compound ID | C00798 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. onsson, Stefan; Ricagno, Stefano; Lindqvist, Ylva; Richards, Nigel G. J. Kinetic and mechanistic characterization of the formyl-CoA transferase from Oxalobacter formigenes. Journal of Biological Chemistry (2004), 279(34), 36003-36012. | | 2. onsson, Stefan; Ricagno, Stefano; Lindqvist, Ylva; Richards, Nigel G. J. Kinetic and mechanistic characterization of the formyl-CoA transferase from Oxalobacter formigenes. Journal of Biological Chemistry (2004), 279(34), 36003-36012. | | 3. Croes K, Casteels M, Asselberghs S, Herdewijn P, Mannaerts GP, Van Veldhoven PP: Formation of a 2-methyl-branched fatty aldehyde during peroxisomal alpha-oxidation. FEBS Lett. 1997 Aug 4;412(3):643-5. | | 4. Croes K, Van Veldhoven PP, Mannaerts GP, Casteels M: Production of formyl-CoA during peroxisomal alpha-oxidation of 3-methyl-branched fatty acids. FEBS Lett. 1997 Apr 28;407(2):197-200. |
|
|---|