| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 05:50:42 UTC |
|---|
| Update Date | 2016-11-09 01:21:19 UTC |
|---|
| Accession Number | CHEM035521 |
|---|
| Identification |
|---|
| Common Name | 4-Hydroxycrotonic acid |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
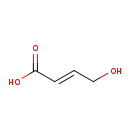
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 4-Hydroxycrotonate | Generator | | trans-4-Hydroxycrotonic acid | ChEMBL, HMDB, MeSH | | trans-4-Hydroxycrotonate | Generator, HMDB | | 4-Hydroxy-2-butenoate | HMDB | | 4-Hydroxy-2-butenoic acid | HMDB | | 4-Hydroxy-crotonic acid | HMDB | | gamma-Hydroxy-crotonic acid | HMDB | | gamma-Hydroxycrotonic acid | HMDB | | 4-Hydroxy-2-butenoic acid, (e)-isomer | MeSH, HMDB | | 4-Hydroxy-2-butenoic acid, (Z)-isomer | MeSH, HMDB | | 4-Hydroxy-2-butenoic acid, sodium salt, (e)-isomer | MeSH, HMDB | | 4-Hydroxy-crotonate | Generator, HMDB |
|
|---|
| Chemical Formula | C4H6O3 |
|---|
| Average Molecular Mass | 102.089 g/mol |
|---|
| Monoisotopic Mass | 102.032 g/mol |
|---|
| CAS Registry Number | 4013-24-5 |
|---|
| IUPAC Name | (2E)-4-hydroxybut-2-enoic acid |
|---|
| Traditional Name | 4-hydroxycrotonic acid |
|---|
| SMILES | OC\C=C\C(O)=O |
|---|
| InChI Identifier | InChI=1S/C4H6O3/c5-3-1-2-4(6)7/h1-2,5H,3H2,(H,6,7)/b2-1+ |
|---|
| InChI Key | RMQJECWPWQIIPW-OWOJBTEDSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as hydroxy fatty acids. These are fatty acids in which the chain bears a hydroxyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty acids and conjugates |
|---|
| Direct Parent | Hydroxy fatty acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Hydroxy fatty acid
- Unsaturated fatty acid
- Straight chain fatty acid
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Primary alcohol
- Organooxygen compound
- Carbonyl group
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4i-9000000000-8fb2f9357973607769fd | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-05fr-9620000000-27fa08857e012141c68c | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-9100000000-c7960a3c750fb74a6bb5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000f-9000000000-2d82bb53f35e31b1c18a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000l-9000000000-79dec3310e408eb4b9c7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-7900000000-238d1b11229e8e8d913f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0ue9-9300000000-3868182d3f88bc2ab925 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a5l-9000000000-34a710d95fdcde102c67 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0ul0-9400000000-55c28706688c4b4dcea8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-05o0-9000000000-57bbaf4883f148891a4e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052r-9000000000-e6590909798fcf08a23f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000l-9000000000-167fa6400146357d8a5d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052o-9000000000-3c4729a0ff72d091d257 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000f-9000000000-81463c00d4e24e43c259 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0003381 |
|---|
| FooDB ID | FDB023160 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | T-HCA |
|---|
| Chemspider ID | 4825947 |
|---|
| ChEBI ID | 553775 |
|---|
| PubChem Compound ID | 6155526 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Niwa T, Maeda K, Asada H, Shibata M, Ohki T, Saito A, Furukawa H: Gas chromatographic-mass spectrometric analysis of organic acids in renal tissue biopsy: identification of 4-hydroxybutyric acid and 4-hydroxy-2-butenoic acid. J Chromatogr. 1982 Jun 11;230(1):1-6. | | 2. Niwa T, Yamamoto N, Asada H, Kawanishi A, Yokoyama M, Maeda K, Ohki T: Ischemic change of organic acids in kidney. J Chromatogr. 1983 Feb 11;272(2):227-32. |
|
|---|