| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 05:49:59 UTC |
|---|
| Update Date | 2016-11-09 01:21:19 UTC |
|---|
| Accession Number | CHEM035509 |
|---|
| Identification |
|---|
| Common Name | Biotripyrrin-b |
|---|
| Class | Small Molecule |
|---|
| Description | Biotripyrrin-a and biotripyrrin-b, bilirubin metabolites, are novel tripyrrole biocompounds and belong to a third group of bile pigments following biliverdin and bilirubin. They are regioisomers of each other. -- Yamaguchi T et al., J Biochem (Tokyo). 1994 Aug;116(2):298-303. PMID 7822247. These metabolites are recognized by an anti-bilirubin monoclonal antibody, 24G7, but are neg. in the diazo reaction. (PubMed ID 9836731 ). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
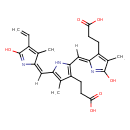
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3-(2-{[(2Z)-3-(2-carboxyethyl)-5-hydroxy-4-methyl-2H-pyrrol-2-ylidene]methyl}-5-{[(2E)-4-ethenyl-5-hydroxy-3-methyl-2H-pyrrol-2-ylidene]methyl}-4-methyl-1H-pyrrol-3-yl)propanoate | Generator |
|
|---|
| Chemical Formula | C25H27N3O6 |
|---|
| Average Molecular Mass | 465.506 g/mol |
|---|
| Monoisotopic Mass | 465.190 g/mol |
|---|
| CAS Registry Number | 158598-18-6 |
|---|
| IUPAC Name | 3-(2-{[(2Z)-3-(2-carboxyethyl)-5-hydroxy-4-methyl-2H-pyrrol-2-ylidene]methyl}-5-{[(2E)-4-ethenyl-5-hydroxy-3-methyl-2H-pyrrol-2-ylidene]methyl}-4-methyl-1H-pyrrol-3-yl)propanoic acid |
|---|
| Traditional Name | 3-(2-{[(2Z)-3-(2-carboxyethyl)-5-hydroxy-4-methylpyrrol-2-ylidene]methyl}-5-{[(2E)-4-ethenyl-5-hydroxy-3-methylpyrrol-2-ylidene]methyl}-4-methyl-1H-pyrrol-3-yl)propanoic acid |
|---|
| SMILES | [H]\C(C1=C(C)C(CCC(O)=O)=C(N1)C(\[H])=C1/N=C(O)C(C)=C1CCC(O)=O)=C1/N=C(O)C(C=C)=C1C |
|---|
| InChI Identifier | InChI=1S/C25H27N3O6/c1-5-15-12(2)19(27-25(15)34)10-18-13(3)16(6-8-22(29)30)20(26-18)11-21-17(7-9-23(31)32)14(4)24(33)28-21/h5,10-11,26H,1,6-9H2,2-4H3,(H,27,34)(H,28,33)(H,29,30)(H,31,32)/b19-10+,21-11- |
|---|
| InChI Key | PMIYFUGIVLGYIZ-YDSWVFJZSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dipyrrins. Dipyrrins are compounds containing two pyrrole rings fused via a methine (-C=) group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pyrroles |
|---|
| Sub Class | Substituted pyrroles |
|---|
| Direct Parent | Dipyrrins |
|---|
| Alternative Parents | |
|---|
| Substituents | - Dipyrrin
- Dicarboxylic acid or derivatives
- Cyclic carboximidic acid
- Heteroaromatic compound
- Carboxylic acid derivative
- Carboxylic acid
- Propargyl-type 1,3-dipolar organic compound
- Organic 1,3-dipolar compound
- Azacycle
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Carbonyl group
- Organopnictogen compound
- Organic nitrogen compound
- Organic oxide
- Hydrocarbon derivative
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-05fs-1101900000-daa980a0c938fb4cb683 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-014i-4000089000-40408d789d1d180c48aa | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0000900000-5e80fb254cbaf8344286 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00dj-0104900000-176df8158ba5be158957 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0200-2938400000-cb8a899917a3bc026213 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03dj-0000900000-cbc81b481d5b63227aff | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0gvk-1000900000-cfee32983a52516212fc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-9111300000-7470247fc7b58a28209c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00kb-0000900000-7bcc33eefe4e1afd19f0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0w90-0107900000-8ec823c438652d0ba4cf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0umi-0309700000-c889b9e40f77166ce88f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0001900000-2c1dbc370e9cbbd65841 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0fk9-0007900000-4b3d779ab9df1ccaa0b7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00di-0009000000-4a4ad2287a6d4545b3bb | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0003324 |
|---|
| FooDB ID | FDB023139 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 59696712 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 131750328 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Yamaguchi T, Shioji I, Sugimoto A, Komoda Y, Nakajima H: Chemical structure of a new family of bile pigments from human urine. J Biochem. 1994 Aug;116(2):298-303. | | 2. Yamaguchi T, Hashizume T, Tanaka M, Nakayama M, Sugimoto A, Ikeda S, Nakajima H, Horio F: Bilirubin oxidation provoked by endotoxin treatment is suppressed by feeding ascorbic acid in a rat mutant unable to synthesize ascorbic acid. Eur J Biochem. 1997 Apr 15;245(2):233-40. | | 3. Shimoharada K, Inoue S, Nakahara M, Kanzaki N, Shimizu S, Kang D, Hamasaki N, Kinoshita S: Urine concentration of biopyrrins: a new marker for oxidative stress in vivo. Clin Chem. 1998 Dec;44(12):2554-5. | | 4. Yamaguchi T, Terakado M, Horio F, Aoki K, Tanaka M, Nakajima H: Role of bilirubin as an antioxidant in an ischemia-reperfusion of rat liver and induction of heme oxygenase. Biochem Biophys Res Commun. 1996 Jun 5;223(1):129-35. | | 5. Yamaguchi T, Horio F, Hashizume T, Tanaka M, Ikeda S, Kakinuma A, Nakajima H: Bilirubin is oxidized in rats treated with endotoxin and acts as a physiological antioxidant synergistically with ascorbic acid in vivo. Biochem Biophys Res Commun. 1995 Sep 5;214(1):11-9. |
|
|---|