| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 05:49:29 UTC |
|---|
| Update Date | 2016-11-09 01:21:19 UTC |
|---|
| Accession Number | CHEM035502 |
|---|
| Identification |
|---|
| Common Name | Prostaglandin G2 |
|---|
| Class | Small Molecule |
|---|
| Description | Not Available |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
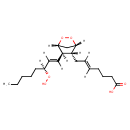
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (5E)-7-[(1R,4S,5R,6R)-6-[(1E,3S)-3-Hydroperoxyoct-1-en-1-yl]-2,3-dioxabicyclo[2.2.1]heptan-5-yl]hept-5-enoate | Generator |
|
|---|
| Chemical Formula | C20H32O6 |
|---|
| Average Molecular Mass | 368.470 g/mol |
|---|
| Monoisotopic Mass | 368.220 g/mol |
|---|
| CAS Registry Number | 51982-36-6 |
|---|
| IUPAC Name | (5E)-7-[(1R,4S,5R,6R)-6-[(1E,3S)-3-hydroperoxyoct-1-en-1-yl]-2,3-dioxabicyclo[2.2.1]heptan-5-yl]hept-5-enoic acid |
|---|
| Traditional Name | (5E)-7-[(1R,4S,5R,6R)-6-[(1E,3S)-3-hydroperoxyoct-1-en-1-yl]-2,3-dioxabicyclo[2.2.1]heptan-5-yl]hept-5-enoic acid |
|---|
| SMILES | [H]\C(CCCC(O)=O)=C(\[H])C[C@@]1([H])[C@]2([H])C[C@@]([H])(OO2)[C@]1([H])C(\[H])=C(/[H])[C@]([H])(CCCCC)OO |
|---|
| InChI Identifier | InChI=1S/C20H32O6/c1-2-3-6-9-15(24-23)12-13-17-16(18-14-19(17)26-25-18)10-7-4-5-8-11-20(21)22/h4,7,12-13,15-19,23H,2-3,5-6,8-11,14H2,1H3,(H,21,22)/b7-4+,13-12+/t15-,16+,17+,18-,19+/m0/s1 |
|---|
| InChI Key | SGUKUZOVHSFKPH-UAAPODJFSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as prostaglandins and related compounds. These are unsaturated carboxylic acids consisting of a 20 carbon skeleton that also contains a five member ring, and are based upon the fatty acid arachidonic acid. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Eicosanoids |
|---|
| Direct Parent | Prostaglandins and related compounds |
|---|
| Alternative Parents | |
|---|
| Substituents | - Prostaglandin skeleton
- Long-chain fatty acid
- Hydroperoxy fatty acid
- Heterocyclic fatty acid
- Fatty acid
- Unsaturated fatty acid
- Ortho-dioxane
- Allylic hydroperoxide
- Ortho-dioxolane
- Dialkyl peroxide
- Hydroperoxide
- Alkyl hydroperoxide
- Oxacycle
- Organoheterocyclic compound
- Peroxol
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0119000000-8d5b5d9014abbb7bac44 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0zmr-3369000000-7084ad1b1cf23bd250b2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0zmu-9400000000-c47e088996d0173f79af | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0009000000-60ad5ed9a4cfd25bbc22 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00r2-2149000000-82226712faf074f3372b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9263000000-054ce347be5aa05b3a84 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 49867498 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|