| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 05:48:53 UTC |
|---|
| Update Date | 2016-11-09 01:21:19 UTC |
|---|
| Accession Number | CHEM035492 |
|---|
| Identification |
|---|
| Common Name | Leukotriene D4 |
|---|
| Class | Small Molecule |
|---|
| Description | Not Available |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
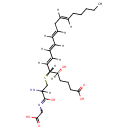
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (5S,6R,7E,9E,11E,14E)-6-({2-amino-2-[(carboxymethyl)-C-hydroxycarbonimidoyl]ethyl}sulfanyl)-5-hydroxyicosa-7,9,11,14-tetraenoate | Generator | | (5S,6R,7E,9E,11E,14E)-6-({2-amino-2-[(carboxymethyl)-C-hydroxycarbonimidoyl]ethyl}sulphanyl)-5-hydroxyicosa-7,9,11,14-tetraenoate | Generator | | (5S,6R,7E,9E,11E,14E)-6-({2-amino-2-[(carboxymethyl)-C-hydroxycarbonimidoyl]ethyl}sulphanyl)-5-hydroxyicosa-7,9,11,14-tetraenoic acid | Generator |
|
|---|
| Chemical Formula | C25H40N2O6S |
|---|
| Average Molecular Mass | 496.660 g/mol |
|---|
| Monoisotopic Mass | 496.261 g/mol |
|---|
| CAS Registry Number | 73836-78-9 |
|---|
| IUPAC Name | (5S,6R,7E,9E,11E,14E)-6-({2-amino-2-[(carboxymethyl)-C-hydroxycarbonimidoyl]ethyl}sulfanyl)-5-hydroxyicosa-7,9,11,14-tetraenoic acid |
|---|
| Traditional Name | (5S,6R,7E,9E,11E,14E)-6-{[2-amino-2-(carboxymethyl-C-hydroxycarbonimidoyl)ethyl]sulfanyl}-5-hydroxyicosa-7,9,11,14-tetraenoic acid |
|---|
| SMILES | [H]\C(CCCCC)=C(\[H])C\C([H])=C(/[H])\C(\[H])=C(/[H])\C(\[H])=C(/[H])[C@@]([H])(SCC([H])(N)C(O)=NCC(O)=O)[C@@]([H])(O)CCCC(O)=O |
|---|
| InChI Identifier | InChI=1S/C25H40N2O6S/c1-2-3-4-5-6-7-8-9-10-11-12-13-16-22(21(28)15-14-17-23(29)30)34-19-20(26)25(33)27-18-24(31)32/h6-7,9-13,16,20-22,28H,2-5,8,14-15,17-19,26H2,1H3,(H,27,33)(H,29,30)(H,31,32)/b7-6+,10-9+,12-11+,16-13+/t20?,21-,22+/m0/s1 |
|---|
| InChI Key | YEESKJGWJFYOOK-JYDLFNMDSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as leukotrienes. These are eicosanoids containing a hydroxyl group attached to the aliphatic chain of an arachidonic acid. Leukotrienes have four double bonds, three (and only three) of which are conjugated. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Eicosanoids |
|---|
| Direct Parent | Leukotrienes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Leukotriene
- Hydroxyeicosatetraenoic acid
- Alpha-dipeptide
- Alpha peptide
- Long-chain fatty acid
- N-acyl-alpha amino acid or derivatives
- N-acyl-alpha-amino acid
- Alpha-amino acid amide
- Cysteine or derivatives
- Alpha-amino acid or derivatives
- Hydroxy fatty acid
- Thia fatty acid
- Dicarboxylic acid or derivatives
- Fatty acid
- Unsaturated fatty acid
- Secondary alcohol
- Secondary carboxylic acid amide
- Amino acid
- Carboxamide group
- Amino acid or derivatives
- Carboxylic acid derivative
- Carboxylic acid
- Dialkylthioether
- Sulfenyl compound
- Thioether
- Amine
- Organic oxygen compound
- Organic nitrogen compound
- Hydrocarbon derivative
- Alcohol
- Organic oxide
- Carbonyl group
- Organopnictogen compound
- Primary amine
- Primary aliphatic amine
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00b9-4103900000-7533869faaff292821f3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-9202100000-f0258aad880858d41a59 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-9001000000-7871ad33e898a45d3462 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0f9t-0203900000-26fadc9296ecde86deb9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0fai-1429100000-7f71783c0c128e2b4cf0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0h90-5911000000-07e316ce5cd008b74e42 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 50989807 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|