| Synonyms | | Value | Source |
|---|
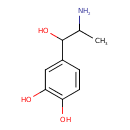
| alpha-Methylnorepinephrine | Kegg | | alpha-Methylnoradrenaline | Kegg | | a-Methylnorepinephrine | Generator | | Α-methylnorepinephrine | Generator | | a-Methylnoradrenaline | Generator | | Α-methylnoradrenaline | Generator | | (+-)-3,4-Dihydroxynorephedrine | HMDB | | (+/-)-3,4-dihydroxynorephedrine | HMDB | | 3,4-Dihydrophenyl-1-amino-2-propanol-1 | HMDB | | Dihydroxyphenylpropanolamine | HMDB | | Dioxynorepinephrine | HMDB | | DL-a-Methylnorepinephrine | HMDB | | DL-alpha-Methylnorepinephrine | HMDB | | Isoadrenaline | HMDB | | L-a-Methylnorepinephrine | HMDB | | L-alpha-Methylnorepinephrine | HMDB | | Levonordefrin | HMDB, MeSH | | Nordefrin | HMDB, MeSH | | Nordefrin hydrochloride, (r*,r*)-(+,-)-isomer | MeSH, HMDB | | Nordefrin, (r*,r*)-isomer | MeSH, HMDB | | 3,4-Dihydroxynorephedrine | MeSH, HMDB | | Cobefrine | MeSH, HMDB | | Corbadrine | MeSH, HMDB | | Nordefrin tartrate, (r*,r*), (r*,r*) isomer | MeSH, HMDB | | 4-(2-amino-1-Hydroxypropyl)-1,2-benzenediol | MeSH, HMDB | | 4-(2-amino-1-Hydroxypropyl)-1,2-benzenediol hydrochloride, (r*,r*)-(+,-)-isomer | MeSH, HMDB | | 4-(2-amino-1-Hydroxypropyl)-1,2-benzenediol hydrochloride, (r*,s*)-(+-)-isomer | MeSH, HMDB | | NeoCobefrin | MeSH, HMDB | | alpha Methylnoradrenaline | MeSH, HMDB | | 3,4 Dihydroxynorephedrine | MeSH, HMDB | | 4-(2-amino-1-Hydroxypropyl)-1,2-benzenediol, (r*,r*)-isomer | MeSH, HMDB | | 4-(2-amino-1-Hydroxypropyl)-1,2-benzenediol, (r*,s*)-isomer | MeSH, HMDB | | Hydrochloride, nordefrin | MeSH, HMDB | | neo-Cobefrin | MeSH, HMDB | | Nordefrin hydrochloride | MeSH, HMDB | | Nordefrin tartrate, (r*,s*), (r*,r*) isomer | MeSH, HMDB | | Nordefrin, (r*,s*)-isomer | MeSH, HMDB | | alpha Methylnorepinephrine | MeSH, HMDB | | 4-(2-amino-1-Hydroxypropyl)-1,2-benzenediol tartrate, (r*,s*), (r*,r*)-isomer | MeSH, HMDB | | Methylnorepinephrine | MeSH, HMDB | | Norephrine | MeSH, HMDB | | 4-(2-amino-1-Hydroxypropyl)-1,2-benzenediol tartrate, (r*,r*), (r*,r*)-isomer | MeSH, HMDB | | neo Cobefrin | MeSH, HMDB | | Nordefrin hydrochloride, (r*,s*)-(+,-)-isomer | MeSH, HMDB |
|
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-000f-9700000000-97a66276892b2b9a6d84 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-0a4i-3009000000-4926ecaf9c8e237270e1 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0159-0900000000-47e648782b51c0926d4c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014j-0900000000-b0d09db351f406d15290 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0f6t-5900000000-d074f2164ac8593850e5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0900000000-677bd5c1c0e6ff820034 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01q9-0900000000-535a6592671a16064650 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4r-1900000000-6417a162c74813d6b283 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-01x0-0900000000-4d329ca34072b59eedd4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0900000000-f3d6884a1d9a0b402eb9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0596-9800000000-0a42555edab27c646a52 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0900000000-fb62819bff82a937e5bc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00kb-2900000000-ef244b4a084824e097b1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0uk9-9600000000-8e21565f2276bece257e | Spectrum |
|
|---|