| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 05:46:49 UTC |
|---|
| Update Date | 2016-11-09 01:21:19 UTC |
|---|
| Accession Number | CHEM035454 |
|---|
| Identification |
|---|
| Common Name | Taurocholic acid 3-sulfate |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
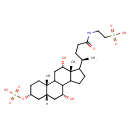
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Cholate glucuronide | Generator | | 7,12-Dihydroxy-3-O-(beta-D-glucopyranosyluronate)-5beta-cholan-24-Oate | HMDB | | 7,12-Dihydroxy-3-O-(beta-D-glucopyranosyluronate)-5beta-cholan-24-Oic acid | HMDB | | 7,12-Dihydroxy-3-O-(beta-delta-glucopyranosyluronate)-5beta-cholan-24-Oate | HMDB | | 7,12-Dihydroxy-3-O-(beta-delta-glucopyranosyluronate)-5beta-cholan-24-Oic acid | HMDB | | Cholic acid 3-O-glucuronide | HMDB | | 7,12-Dihydroxy-3-O-(beta-D-glucopyranosyluronate)-5 beta-cholan-24-Oate | HMDB | | 3-Sulfocholyltaurine | HMDB | | Taurocholic acid 3-sulphate | HMDB | | Taurocholic acid 3a-sulfate | HMDB | | Taurocholic acid 3a-sulphate | HMDB | | (4R)-4-[(2S,5R,7R,9R,15R,16S)-9,16-Dihydroxy-2,15-dimethyl-5-(sulfooxy)tetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadecan-14-yl]-N-(2-sulfoethyl)pentanimidate | Generator, HMDB | | (4R)-4-[(2S,5R,7R,9R,15R,16S)-9,16-Dihydroxy-2,15-dimethyl-5-(sulphooxy)tetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadecan-14-yl]-N-(2-sulphoethyl)pentanimidate | Generator, HMDB | | (4R)-4-[(2S,5R,7R,9R,15R,16S)-9,16-Dihydroxy-2,15-dimethyl-5-(sulphooxy)tetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadecan-14-yl]-N-(2-sulphoethyl)pentanimidic acid | Generator, HMDB | | Taurocholate 3-sulfate | Generator | | Taurocholate 3-sulphate | Generator | | Taurocholic acid 3-sulfuric acid | Generator | | Taurocholic acid 3-sulphuric acid | Generator |
|
|---|
| Chemical Formula | C26H45NO10S2 |
|---|
| Average Molecular Mass | 595.766 g/mol |
|---|
| Monoisotopic Mass | 595.248 g/mol |
|---|
| CAS Registry Number | 67030-62-0 |
|---|
| IUPAC Name | 2-[(4R)-4-[(2S,5R,7R,9R,15R,16S)-9,16-dihydroxy-2,15-dimethyl-5-(sulfooxy)tetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadecan-14-yl]pentanamido]ethane-1-sulfonic acid |
|---|
| Traditional Name | 2-[(4R)-4-[(2S,5R,7R,9R,15R,16S)-9,16-dihydroxy-2,15-dimethyl-5-(sulfooxy)tetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadecan-14-yl]pentanamido]ethanesulfonic acid |
|---|
| SMILES | [H][C@]12C[C@@H](O)C3C4CCC([C@H](C)CCC(=O)NCCS(O)(=O)=O)[C@@]4(C)[C@@H](O)CC3[C@@]1(C)CC[C@H](C2)OS(O)(=O)=O |
|---|
| InChI Identifier | InChI=1S/C26H45NO10S2/c1-15(4-7-23(30)27-10-11-38(31,32)33)18-5-6-19-24-20(14-22(29)26(18,19)3)25(2)9-8-17(37-39(34,35)36)12-16(25)13-21(24)28/h15-22,24,28-29H,4-14H2,1-3H3,(H,27,30)(H,31,32,33)(H,34,35,36)/t15-,16+,17-,18?,19?,20?,21-,22+,24?,25+,26-/m1/s1 |
|---|
| InChI Key | LKTXOQJJFLAZRW-XVBIMYNNSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as steroid glucuronide conjugates. These are sterol lipids containing a glucuronide moiety linked to the steroid skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Steroidal glycosides |
|---|
| Direct Parent | Steroid glucuronide conjugates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Steroid-glucuronide-skeleton
- Dihydroxy bile acid, alcohol, or derivatives
- Hydroxy bile acid, alcohol, or derivatives
- Bile acid, alcohol, or derivatives
- 7-hydroxysteroid
- 12-hydroxysteroid
- Hydroxysteroid
- 1-o-glucuronide
- O-glucuronide
- Glucuronic acid or derivatives
- Hexose monosaccharide
- Glycosyl compound
- O-glycosyl compound
- Beta-hydroxy acid
- Dicarboxylic acid or derivatives
- Hydroxy acid
- Pyran
- Oxane
- Monosaccharide
- Cyclic alcohol
- Secondary alcohol
- Organoheterocyclic compound
- Oxacycle
- Acetal
- Carboxylic acid
- Carboxylic acid derivative
- Polyol
- Alcohol
- Hydrocarbon derivative
- Carbonyl group
- Organic oxygen compound
- Organic oxide
- Organooxygen compound
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0gc0-0156290000-44d206389b2fdd75e495 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0uxr-2017329000-8cec864e79df15ac12b7 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS ("Taurocholic acid 3-sulfate,1TMS,#1" TMS) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_4) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_5) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_4) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_5) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_6) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_7) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_8) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_9) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_10) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_4) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_5) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_3) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0200590000-1ea1283870fd76cdfff2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-057j-1701920000-080dde21bbe7e7c279e5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004l-6803920000-1880757f4111100bf8c2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-2000390000-2d4944cc4735fb398571 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000t-5300970000-38c7359d7957cf3c5692 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0089-9501300000-b30f90843c07724ff22f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-002b-0000190000-a0dfb668eac941bf8ce1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-054k-4920560000-f6211de922794e5c8c8b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-02ar-4495200000-71f16b20f8b848259f9a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0000090000-7867a8da40ba0878afce | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-1000090000-0bf81ac66ff94d5943d2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-9120030000-115e4e45bc7a09940bc6 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0002577 |
|---|
| FooDB ID | FDB023026 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | 6714 |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 13628368 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 21252309 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|