| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 05:43:53 UTC |
|---|
| Update Date | 2016-11-09 01:21:18 UTC |
|---|
| Accession Number | CHEM035399 |
|---|
| Identification |
|---|
| Common Name | 4a-Hydroxytetrahydrobiopterin |
|---|
| Class | Small Molecule |
|---|
| Description | A tetrahydropterin that is 5,6,7,8-tetrahydrobiopterin carrying an additional hydroxy substituent at the 4a-position. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
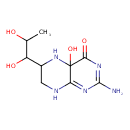
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 4a-Hydroxy-5,6,4,8-tetrahydrobiopterin | ChEBI | | (6R)-6-(L-erythro-1,2-Dihydroxypropyl)-5,6,7,8-tetrahydro-4a-hydroxypterin | ChEBI, HMDB | | 2-amino-6-(1,2-Dihydroxypropyl)-4a-hydroxy-5,6,7,8-tetrahydropteridin-4(4ah)-one | HMDB | | 2-amino-6-(1,2-Dihydroxypropyl)-5,6,7,8-tetrahydro-4a-hydroxy-4(4ah)-pteridinone | HMDB | | 2-amino-6-[(1R,2S)-1,2-Dihydroxypropyl]-4a-hydroxy-1,5,6,7-tetrahydropteridin-4-one | HMDB | | 4a-Hydroxy-5,6,7,8-tetrahydrobiopterin | HMDB | | 4alpha-Hydroxytetrahydrobiopterin | HMDB | | 4a-HTHB | MeSH, HMDB |
|
|---|
| Chemical Formula | C9H14N4O3 |
|---|
| Average Molecular Mass | 226.233 g/mol |
|---|
| Monoisotopic Mass | 226.107 g/mol |
|---|
| CAS Registry Number | 70110-58-6 |
|---|
| IUPAC Name | 2-amino-6-(1,2-dihydroxypropyl)-4a-hydroxy-4,4a,5,6,7,8-hexahydropteridin-4-one |
|---|
| Traditional Name | 4a-hydroxytetrahydrobiopterin |
|---|
| SMILES | C[C@H](O)C(O)[C@@H]1CNC2=C(N1)C(=O)N=CN2 |
|---|
| InChI Identifier | InChI=1S/C9H14N4O3/c1-4(14)7(15)5-2-10-8-6(13-5)9(16)12-3-11-8/h3-5,7,13-15H,2H2,1H3,(H2,10,11,12,16)/t4-,5-,7?/m0/s1 |
|---|
| InChI Key | XHZMOKNFPZDZBZ-CNGBTNQNSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as biopterins and derivatives. These are coenzymes containing a 2-amino-pteridine-4-one derivative. They are mainly synthesized in several parts of the body, including the pineal gland. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pteridines and derivatives |
|---|
| Sub Class | Pterins and derivatives |
|---|
| Direct Parent | Biopterins and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Biopterin
- Hydropyrimidine
- 1,2,5,6-tetrahydropyrimidine
- Imidolactam
- 1,2-diol
- Secondary alcohol
- Alkanolamine
- Amidine
- Carboxylic acid amidine
- Secondary aliphatic amine
- Azacycle
- Secondary amine
- Carboximidamide
- Propargyl-type 1,3-dipolar organic compound
- Organic 1,3-dipolar compound
- Organopnictogen compound
- Hydrocarbon derivative
- Imine
- Organonitrogen compound
- Organooxygen compound
- Alcohol
- Amine
- Organic oxygen compound
- Organic nitrogen compound
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-001i-4930000000-89d1b72adfc56cf4e3fd | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-0zfr-5293600000-e1bc928bae70ea520f93 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4l-0090000000-364ec2c50acd77d83ef0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00gl-0690000000-f15f00b5ba6b8f2959b9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0005-9720000000-7a2198134bfe87159831 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0bt9-2290000000-43723819afb4c98b5ceb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-9210000000-fa8eb33fdfed731f1c82 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9100000000-c550affb7745a16d59b4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0090000000-2e38d752208e421512bf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4l-0390000000-8910ee8de849000f68e5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-5900000000-b0f630ab5b90aa052132 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0090000000-195221ba76407cd71968 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01ox-1690000000-492a38357ab54f3370a4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0296-9820000000-44ae87e7d45d04a8c51a | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0304198 |
|---|
| FooDB ID | FDB030533 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | CPD-5881 |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 114908 |
|---|
| ChEBI ID | 15374 |
|---|
| PubChem Compound ID | 129803 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|