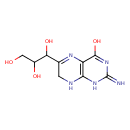
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-03dr-9220000000-602126fb02cae16969f6 | Spectrum |
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-0aw9-3292200000-e6b52358cd355e939ab0 | Spectrum |
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum |
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum |
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_4_31) - 70eV, Positive | Not Available | Spectrum |
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_4_32) - 70eV, Positive | Not Available | Spectrum |
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_4_33) - 70eV, Positive | Not Available | Spectrum |
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_4_34) - 70eV, Positive | Not Available | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 2V, positive | splash10-0a4i-0090000000-c40ef232ef8c10af6b4a | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 3V, positive | splash10-0a4i-0090000000-40779978322477cf298a | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 5V, positive | splash10-0a4i-0090000000-e49838b4e8d652d72bdf | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 7V, positive | splash10-0a4r-0390000000-b43231e2e78443a996d7 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 10V, positive | splash10-014i-0920000000-5f6d06fef1bff433cb74 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 12V, positive | splash10-014i-0900000000-1f59c6362ecd3bf94b79 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 16V, positive | splash10-014i-0900000000-5299b47c47609ee94a30 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 20V, positive | splash10-014i-1900000000-f31d91ebf5d2ad43354c | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 24V, positive | splash10-01ba-3900000000-4d3e2458159bb9f65ce6 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 28V, positive | splash10-00xs-6900000000-86a6eac4069432a18ae5 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 33V, positive | splash10-00r7-9600000000-971378f9c783f6d800ff | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 38V, positive | splash10-014l-9200000000-687b58605bb70fc727db | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 46V, positive | splash10-014i-9000000000-b01418dfcc77acb66b6e | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - n/a 17V, positive | splash10-014i-0900000000-5da76eb2e54c7dcf9422 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - n/a 17V, positive | splash10-014i-0900000000-df54ce2cbff970a469f4 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - n/a 17V, positive | splash10-004i-0900000000-d6756ef30d881fb25685 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - n/a 17V, positive | splash10-001l-0900000000-e8baecab87622e9340c6 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - n/a 17V, positive | splash10-0w29-0900000000-4952089b8c8b79285dea | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - n/a 17V, positive | splash10-0ab9-0690000000-1f127d1244a709e9919f | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0090000000-e5dad4abb411efa4feb6 | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01p9-1790000000-449cabdd2508eefabccc | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03dj-3900000000-b2abed21008ce5fb01bd | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udl-1390000000-1e1c831e7f68ac77caed | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-4950000000-948e3120b1a4f548982b | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-9300000000-581b6b2fd47ffbefaa0c | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |