| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 05:39:21 UTC |
|---|
| Update Date | 2016-11-09 01:21:17 UTC |
|---|
| Accession Number | CHEM035314 |
|---|
| Identification |
|---|
| Common Name | Tiglyl-CoA |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
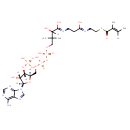
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (e)-2-Methylcrotonoyl-CoA | HMDB | | (e)-2-Methylcrotonoyl-coenzyme A | HMDB | | 2-Methylbut-2-enoyl-CoA | HMDB | | 2-Methylbut-2-enoyl-coenzyme A | HMDB | | 2-Methylcrotanoyl-CoA | HMDB | | 2-Methylcrotanoyl-coenzyme A | HMDB | | 2-Methylcrotonoyl-CoA | HMDB | | 2-Methylcrotonoyl-coenzyme A | HMDB | | 2-Methylcrotonyl-CoA | HMDB | | 2-Methylcrotonyl-coenzyme A | HMDB | | Methylcrotonoyl-CoA | HMDB | | Methylcrotonoyl-coenzyme A | HMDB | | Methylcrotonyl-CoA | HMDB | | Methylcrotonyl-coenzyme A | HMDB | | Tigloyl-CoA | HMDB | | Tigloyl-coenzyme A | HMDB | | Tiglyl-coenzyme A | HMDB | | trans-2-Methylbut-2-enoyl-CoA | HMDB | | trans-2-Methylbut-2-enoyl-coenzyme A | HMDB |
|
|---|
| Chemical Formula | C26H42N7O17P3S |
|---|
| Average Molecular Mass | 849.635 g/mol |
|---|
| Monoisotopic Mass | 849.157 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | {[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-2-({[hydroxy({hydroxy[3-hydroxy-2,2-dimethyl-3-({2-[(2-{[(2E)-2-methylbut-2-enoyl]sulfanyl}ethyl)carbamoyl]ethyl}carbamoyl)propoxy]phosphoryl}oxy)phosphoryl]oxy}methyl)oxolan-3-yl]oxy}phosphonic acid |
|---|
| Traditional Name | [(2R,3S,4R,5R)-5-(6-aminopurin-9-yl)-4-hydroxy-2-({[hydroxy([hydroxy(3-hydroxy-2,2-dimethyl-3-({2-[(2-{[(2E)-2-methylbut-2-enoyl]sulfanyl}ethyl)carbamoyl]ethyl}carbamoyl)propoxy)phosphoryl]oxy)phosphoryl]oxy}methyl)oxolan-3-yl]oxyphosphonic acid |
|---|
| SMILES | C\C=C(/C)C(=O)SCCNC(=O)CCNC(=O)C(O)C(C)(C)COP(O)(=O)OP(O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP(O)(O)=O)N1C=NC2=C(N)N=CN=C12 |
|---|
| InChI Identifier | InChI=1S/C26H42N7O17P3S/c1-5-14(2)25(38)54-9-8-28-16(34)6-7-29-23(37)20(36)26(3,4)11-47-53(44,45)50-52(42,43)46-10-15-19(49-51(39,40)41)18(35)24(48-15)33-13-32-17-21(27)30-12-31-22(17)33/h5,12-13,15,18-20,24,35-36H,6-11H2,1-4H3,(H,28,34)(H,29,37)(H,42,43)(H,44,45)(H2,27,30,31)(H2,39,40,41)/b14-5+/t15-,18-,19-,20?,24-/m1/s1 |
|---|
| InChI Key | PMWATMXOQQZNBX-APMDNKNFSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as ortho cresols. These are organic compounds containing an ortho-cresol moiety, which consists of a benzene bearing one hydroxyl group at ring positions 1 and 2, respectively. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Phenols |
|---|
| Sub Class | Cresols |
|---|
| Direct Parent | Ortho cresols |
|---|
| Alternative Parents | |
|---|
| Substituents | - O-cresol
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Toluene
- Monocyclic benzene moiety
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-1912000120-81b21095705a1243f37e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-1912000000-62328cd733089510fd34 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-2911000000-95f748a6c71560aeb958 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-003r-9830140640-81896860f616d5db847b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-003r-5910010010-e384bb18aba2816a9a63 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-057i-6900100000-a10b6138b97c5a8d8893 | Spectrum | | MS | Mass Spectrum (Electron Ionization) | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0002054 |
|---|
| FooDB ID | FDB022819 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | 41674 |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | 448 |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Tiglyl-CoA |
|---|
| Chemspider ID | 4444187 |
|---|
| ChEBI ID | 15478 |
|---|
| PubChem Compound ID | 5280564 |
|---|
| Kegg Compound ID | C03345 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | ECMDB02054 |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|