| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 05:37:52 UTC |
|---|
| Update Date | 2016-11-09 01:21:17 UTC |
|---|
| Accession Number | CHEM035279 |
|---|
| Identification |
|---|
| Common Name | gamma-CEHC |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
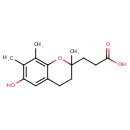
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| g-CEHC | Generator | | Γ-cehc | Generator | | 3-(6-Hydroxy-2,7,8-trimethyl-3,4-dihydro-2H-1-benzopyran-2-yl)propanoate | HMDB | | S-LLU-alpha | HMDB | | LLU-alpha | HMDB | | 3-(2,7,8-Trimethyl-3,4-dihydro-2H-chromen-2-yl)propanoate | HMDB | | (±)-llu-α | HMDB | | (±)-llu-alpha | HMDB | | 2,7,8-Trimethyl-2-(β-carboxyethyl)-6-hydroxychroman | HMDB | | 2,7,8-Trimethyl-2-(beta-carboxyethyl)-6-hydroxychroman | HMDB | | γ-Carboxyethyl hydroxychroman | HMDB | | γ-Carboxyethyl-hydroxychroman | HMDB | | gamma-Carboxyethyl hydroxychroman | HMDB | | gamma-Carboxyethyl-hydroxychroman | HMDB | | 3'-Carboxychromanol | HMDB | | gamma-CEHC | MeSH |
|
|---|
| Chemical Formula | C15H20O3 |
|---|
| Average Molecular Mass | 248.318 g/mol |
|---|
| Monoisotopic Mass | 248.141 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 3-(6-hydroxy-2,7,8-trimethyl-3,4-dihydro-2H-1-benzopyran-2-yl)propanoic acid |
|---|
| Traditional Name | 3-(6-hydroxy-2,7,8-trimethyl-3,4-dihydro-1-benzopyran-2-yl)propanoic acid |
|---|
| SMILES | CC1=CC=C2CCC(C)(CCC(O)=O)OC2=C1C |
|---|
| InChI Identifier | InChI=1S/C15H20O3/c1-10-4-5-12-6-8-15(3,9-7-13(16)17)18-14(12)11(10)2/h4-5H,6-9H2,1-3H3,(H,16,17) |
|---|
| InChI Key | WTGHQIKMADLFAH-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 1-benzopyrans. These are organic aromatic compounds that 1-benzopyran, a bicyclic compound made up of a benzene ring fused to a pyran, so that the oxygen atom is at the 1-position. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Benzopyrans |
|---|
| Sub Class | 1-benzopyrans |
|---|
| Direct Parent | 1-benzopyrans |
|---|
| Alternative Parents | |
|---|
| Substituents | - 1-benzopyran
- 1-hydroxy-2-unsubstituted benzenoid
- Alkyl aryl ether
- Benzenoid
- Oxacycle
- Monocarboxylic acid or derivatives
- Ether
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0390000000-b666254f822f684d0f90 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-2920000000-bb8603bd0385ca985211 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05r9-5900000000-5e06b4301c1de7edc79a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0490000000-f7808533b982dc68faac | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0f6t-0930000000-bd68d5cb372a6bcdc833 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fdk-4900000000-8472a89e5baa2c694314 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0090000000-3a627b5dee80a85c1360 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-1290000000-91128d74296130540d8c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0iki-2950000000-0c65f7f58462d6926552 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0001931 |
|---|
| FooDB ID | FDB022748 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 117455 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 15887183 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|