| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 05:37:10 UTC |
|---|
| Update Date | 2016-11-09 01:21:16 UTC |
|---|
| Accession Number | CHEM035266 |
|---|
| Identification |
|---|
| Common Name | 1,5-Dihydroriboflavin |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
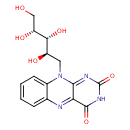
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 4a,5-Dihydroriboflavin | HMDB | | 4a,5-Dihydroriboflavine | HMDB | | 7,8-Dimethyl-10-(D-ribo-2,3,4,5-tetrahydroxypentyl)-4a,5-dihydroisoalloxazine | HMDB | | 7,8-Dimethyl-10-(D-ribo-2,3,4,5-tetrahydroxypentyl)-5,10-dihydrobenzo[g]pteridine-2,4(3H,4ah)-dione | HMDB | | Reduced riboflavin | HMDB |
|
|---|
| Chemical Formula | C17H22N4O6 |
|---|
| Average Molecular Mass | 378.380 g/mol |
|---|
| Monoisotopic Mass | 378.154 g/mol |
|---|
| CAS Registry Number | 101652-10-2 |
|---|
| IUPAC Name | 10-[(2R,3R,4R)-2,3,4,5-tetrahydroxypentyl]-2H,3H,4H,10H-benzo[g]pteridine-2,4-dione |
|---|
| Traditional Name | 10-[(2R,3R,4R)-2,3,4,5-tetrahydroxypentyl]-3H-benzo[g]pteridine-2,4-dione |
|---|
| SMILES | CC1=C(C)C=C2N(C[C@H](O)[C@H](O)[C@H](O)CO)C3=C(NC2=C1)C(=O)NC(=O)N3 |
|---|
| InChI Identifier | InChI=1S/C17H22N4O6/c1-7-3-9-10(4-8(7)2)21(5-11(23)14(25)12(24)6-22)15-13(18-9)16(26)20-17(27)19-15/h3-4,11-12,14,18,22-25H,5-6H2,1-2H3,(H2,19,20,26,27)/t11-,12+,14-/m0/s1 |
|---|
| InChI Key | SGSVWAYHEWEQET-SCRDCRAPSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as alloxazines and isoalloxazines. These are organic compounds comprising the (iso)alloxazine structure (Benzo[g]pteridine-2,4-dione). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pteridines and derivatives |
|---|
| Sub Class | Alloxazines and isoalloxazines |
|---|
| Direct Parent | Alloxazines and isoalloxazines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Isoalloxazine
- Diazanaphthalene
- Quinoxaline
- Pyrimidone
- Pyrazine
- Pyrimidine
- Benzenoid
- Heteroaromatic compound
- Vinylogous amide
- Secondary alcohol
- Lactam
- Polyol
- Azacycle
- Alcohol
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Primary alcohol
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0bwi-8094000000-f43f7112ee283826153b | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-00di-3042269000-7f5ae8c8ce0af8c53f55 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_3_7) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_4_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_4_5) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS ("Riboflavin reduced,3TBDMS,#7" TMS) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000t-0029000000-bc104c9d050e033b69d4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01t9-4091000000-afc0f707ee1ce75b867c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004i-2190000000-570c6cbcf04a46a68c76 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udl-4097000000-b676ae8b47eab2fb18c7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-9000000000-f5254591b51a0d846765 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9010000000-0abc9b0d47dd58c290bd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0029000000-01cbdc02a72aeccc6223 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01ta-0098000000-708769959f1af25b7e78 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00b9-0590000000-bc32c00ac0788074d256 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0092000000-f9ec2194483db51c8410 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03fr-1090000000-71092bfa8904438a88ac | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0ffx-6590000000-eca89bd048fc24f150a8 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0001557 |
|---|
| FooDB ID | FDB112174 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | 6322 |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 17216138 |
|---|
| ChEBI ID | 169293 |
|---|
| PubChem Compound ID | 22833571 |
|---|
| Kegg Compound ID | C01007 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | ECMDB21279 |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|