| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 05:36:44 UTC |
|---|
| Update Date | 2016-11-09 01:21:16 UTC |
|---|
| Accession Number | CHEM035257 |
|---|
| Identification |
|---|
| Common Name | 5,10-Methylenetetrahydrofolic acid |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
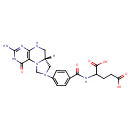
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (6R)-5,10-Methylenetetrahydrofolate | Kegg | | (6R)-5,10-Methylenetetrahydrofolic acid | Generator | | 5,10-Methenyltetrahydropteroylglutamate | HMDB | | 5,10-Methylene-6-hydrofolate | HMDB | | 5,10-Methylene-6-hydrofolic acid | HMDB | | 5,10-Methylenetetrahydrofolate | HMDB | | 5,10-Methylenetetrahydrofolic acid | HMDB | | N5>,N10-methylenetetrahydrofolate | HMDB | | 5,10-Methylene-5,6,7,8-tetrahydrofolate | HMDB | | Tetrahydromethylenefolate | HMDB | | 5,10-Methylenetetrahydrofolate, (D-glu)-isomer | HMDB | | CH2H4Folate | HMDB | | 5,10-Methylenetetrahydrofolate monohydrochloride, (L-glu)-isomer | HMDB | | 5,10-Methylenetetrahydrofolate, (L-glu)-(S)-isomer | HMDB |
|
|---|
| Chemical Formula | C20H23N7O6 |
|---|
| Average Molecular Mass | 457.440 g/mol |
|---|
| Monoisotopic Mass | 457.171 g/mol |
|---|
| CAS Registry Number | 3432-99-3 |
|---|
| IUPAC Name | 2-({4-[(6aR)-1-hydroxy-3-imino-3H,4H,5H,6H,6aH,7H,8H,9H-imidazo[1,5-f]pteridin-8-yl]phenyl}formamido)pentanedioic acid |
|---|
| Traditional Name | 2-({4-[(6aR)-1-hydroxy-3-imino-4H,5H,6H,6aH,7H,9H-imidazo[1,5-f]pteridin-8-yl]phenyl}formamido)pentanedioic acid |
|---|
| SMILES | [H][C@@]12CN(CN1C1=C(NC2)N=C(N)NC1=O)C1=CC=C(C=C1)C(=O)NC(CCC(O)=O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C20H23N7O6/c21-20-24-16-15(18(31)25-20)27-9-26(8-12(27)7-22-16)11-3-1-10(2-4-11)17(30)23-13(19(32)33)5-6-14(28)29/h1-4,12-13H,5-9H2,(H,23,30)(H,28,29)(H,32,33)(H4,21,22,24,25,31)/t12-,13?/m1/s1 |
|---|
| InChI Key | QYNUQALWYRSVHF-PZORYLMUSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as tetrahydrofolic acids. These are heterocyclic compounds based on the 5,6,7,8-tetrahydropteroic acid skeleton conjugated with at least one L-glutamic acid unit. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pteridines and derivatives |
|---|
| Sub Class | Pterins and derivatives |
|---|
| Direct Parent | Tetrahydrofolic acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Tetrahydrofolic acid
- Glutamic acid or derivatives
- N-acyl-alpha amino acid or derivatives
- N-acyl-alpha-amino acid
- Hippuric acid
- Hippuric acid or derivatives
- Phenylimidazolidine
- Alpha-amino acid or derivatives
- Benzamide
- Benzoic acid or derivatives
- Imidazopyrazine
- Benzoyl
- Dialkylarylamine
- Aniline or substituted anilines
- Pyrimidone
- Aminopyrimidine
- Secondary aliphatic/aromatic amine
- Benzenoid
- Pyrimidine
- Monocyclic benzene moiety
- Dicarboxylic acid or derivatives
- Heteroaromatic compound
- Vinylogous amide
- Imidazolidine
- Secondary carboxylic acid amide
- Amino acid or derivatives
- Amino acid
- Carboxamide group
- Secondary amine
- Azacycle
- Carboxylic acid
- Carboxylic acid derivative
- Primary amine
- Organic nitrogen compound
- Carbonyl group
- Hydrocarbon derivative
- Amine
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Organonitrogen compound
- Organooxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-03di-1635900000-4b2ed6618451a7e931b1 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-001i-2129050000-80e96fb3203322200945 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-08fu-0003900000-ce1f75a98c0216d169e2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0229400000-c8c29bf14ef80a73f864 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-02u3-1192000000-ab556f50884c7b4fdcbe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0001900000-6ba3ddeb1f652fdc6b6b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-08fr-1225900000-82b75909f87dfeb54211 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9553000000-5aca24c246430955de51 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0bt9-0006900000-dd6e195db40930799024 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0109200000-aa6336e6c1c41c98d390 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01q9-1695000000-8b9546bbad524392a35b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000f-0009800000-1ef964ba403eb61f6bf0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01w3-1407900000-f4b4d0db119b458049fb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udl-5935200000-5fa8390660d5a558ac8b | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0001533 |
|---|
| FooDB ID | FDB022675 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00007250 |
|---|
| BiGG ID | 34022 |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | 6304 |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 388320 |
|---|
| ChEBI ID | 15636 |
|---|
| PubChem Compound ID | 439175 |
|---|
| Kegg Compound ID | C00143 |
|---|
| YMDB ID | YMDB00260 |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|