| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 05:36:28 UTC |
|---|
| Update Date | 2016-11-09 01:21:16 UTC |
|---|
| Accession Number | CHEM035251 |
|---|
| Identification |
|---|
| Common Name | Thiamine triphosphate |
|---|
| Class | Small Molecule |
|---|
| Description | A thiamine phosphate that is thiamine(1+) in which the hydroxy group is replaced by a triphosphate group. It is found in low amounts in most vertebrate tissues and can phosphorylate certain proteins. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
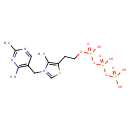
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3-[(4-Amino-2-methyl-5-pyrimidinyl)methyl]-4-methyl-5-(4,6,8,8-trihydroxy-4,6,8-trioxido-3,5,7-trioxa-4,6,8-triphosphaoct-1-yl)-thiazolium | ChEBI | | 3-[(4-Amino-2-methylpyrimidin-5-yl)methyl]-5-{2-[(hydroxy{[hydroxy(phosphonooxy)phosphoryl]oxy}phosphoryl)oxy]ethyl}-4-methyl-1,3-thiazol-3-ium | ChEBI | | Thiamin triphosphate | ChEBI | | Thiamine triphosphoric acid ester | ChEBI | | THTP | ChEBI | | TTP | ChEBI | | Thiamin triphosphoric acid | Generator | | Thiamine triphosphate ester | Generator | | Thiamine triphosphoric acid | Generator | | Triphosphate, thiamin | MeSH, HMDB | | Triphosphate, thiamine | MeSH, HMDB |
|
|---|
| Chemical Formula | C12H20N4O10P3S |
|---|
| Average Molecular Mass | 505.294 g/mol |
|---|
| Monoisotopic Mass | 505.011 g/mol |
|---|
| CAS Registry Number | 3475-65-8 |
|---|
| IUPAC Name | 3-[(4-amino-2-methylpyrimidin-5-yl)methyl]-5-(2-{[hydroxy({[hydroxy(phosphonooxy)phosphoryl]oxy})phosphoryl]oxy}ethyl)-4-methyl-1,3-thiazol-3-ium |
|---|
| Traditional Name | thiamin triphosphate |
|---|
| SMILES | CC1=C(CCOP(O)(=O)OP(O)(=O)OP(O)(O)=O)SC=[N+]1CC1=C(N)N=C(C)N=C1 |
|---|
| InChI Identifier | InChI=1S/C12H19N4O10P3S/c1-8-11(30-7-16(8)6-10-5-14-9(2)15-12(10)13)3-4-24-28(20,21)26-29(22,23)25-27(17,18)19/h5,7H,3-4,6H2,1-2H3,(H5-,13,14,15,17,18,19,20,21,22,23)/p+1 |
|---|
| InChI Key | IWLROWZYZPNOFC-UHFFFAOYSA-O |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as thiamine phosphates. These are thiamine derivatives in which the hydroxyl group of the ethanol moiety is substituted by a phosphate group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Diazines |
|---|
| Sub Class | Pyrimidines and pyrimidine derivatives |
|---|
| Direct Parent | Thiamine phosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Thiamine-phosphate
- 4,5-disubstituted 1,3-thiazole
- Aminopyrimidine
- Monoalkyl phosphate
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Alkyl phosphate
- Imidolactam
- Azole
- Thiazole
- Heteroaromatic compound
- Azacycle
- Amine
- Hydrocarbon derivative
- Primary amine
- Organic oxide
- Organooxygen compound
- Organonitrogen compound
- Organopnictogen compound
- Organic nitrogen compound
- Organic oxygen compound
- Organic cation
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-05bb-9674400000-b85f96fa15a79b807f57 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-1100090000-be999be10fb2a29960f4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0k9i-3231970000-d7309409890edb94fbbd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052b-9820100000-7518cd452597a739fd3d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0000090000-7ac188b5e05c4f14c656 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-9000120000-50842bfa40a49ea4ede5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9320000000-6249c4331931f95b126c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0300190000-c9dd820f036fb97ca251 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00dj-0588940000-745fc053a6eb56c314d7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-0910000000-2071dd01d569c75d980f | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0001512 |
|---|
| FooDB ID | FDB022666 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | 41046 |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | 3586 |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Thiamine triphosphate |
|---|
| Chemspider ID | 496 |
|---|
| ChEBI ID | 9534 |
|---|
| PubChem Compound ID | 511 |
|---|
| Kegg Compound ID | C03028 |
|---|
| YMDB ID | YMDB00775 |
|---|
| ECMDB ID | M2MDB005722 |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|