| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 05:35:31 UTC |
|---|
| Update Date | 2016-11-09 01:21:16 UTC |
|---|
| Accession Number | CHEM035227 |
|---|
| Identification |
|---|
| Common Name | Biotin amide |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
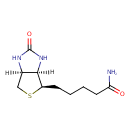
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 5-[(3AS,6R,6ar)-2-hydroxy-1H,3ah,4H,6H,6ah-thieno[3,4-D]imidazol-6-yl]pentanimidate | Generator, HMDB |
|
|---|
| Chemical Formula | C10H17N3O2S |
|---|
| Average Molecular Mass | 243.326 g/mol |
|---|
| Monoisotopic Mass | 243.104 g/mol |
|---|
| CAS Registry Number | 6929-42-6 |
|---|
| IUPAC Name | 5-[(3aR,4R,6aS)-2-oxo-hexahydro-1H-thieno[3,4-d]imidazolidin-4-yl]pentanamide |
|---|
| Traditional Name | 5-[(3aR,4R,6aS)-2-oxo-hexahydrothieno[3,4-d]imidazolidin-4-yl]pentanamide |
|---|
| SMILES | [H][C@@]12CS[C@H](CCCCC(N)=O)[C@]1([H])NC(=O)N2 |
|---|
| InChI Identifier | InChI=1S/C10H17N3O2S/c11-8(14)4-2-1-3-7-9-6(5-16-7)12-10(15)13-9/h6-7,9H,1-5H2,(H2,11,14)(H2,12,13,15)/t6-,7-,9-/m1/s1 |
|---|
| InChI Key | XFLVBMBRLSCJAI-ZXFLCMHBSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as biotin and derivatives. These are organic compounds containing a ureido (tetrahydroimidizalone) ring fused with a tetrahydrothiophene ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Biotin and derivatives |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Biotin and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Biotin_derivative
- Thienoimidazolidine
- Fatty amide
- Imidazolidinone
- Fatty acyl
- Thiophene
- Thiolane
- Imidazolidine
- Carboxamide group
- Urea
- Primary carboxylic acid amide
- Carbonic acid derivative
- Azacycle
- Dialkylthioether
- Thioether
- Carboxylic acid derivative
- Organopnictogen compound
- Organonitrogen compound
- Organic nitrogen compound
- Organooxygen compound
- Organic oxygen compound
- Carbonyl group
- Hydrocarbon derivative
- Organic oxide
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0005-9520000000-51917f0c6418688ca18f | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004l-0090000000-ee632e902d02574234fa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-1490000000-e498ef37a7c5e9770642 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-053v-9600000000-f999e8de54094b58e165 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0190000000-43c6964de1c7149ffeb3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0007-6950000000-055ec27fc755f5f53db3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9000000000-37240324d52248632db0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004l-0090000000-4be63d0dd445aeb87350 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-0190000000-5972c30db84501e53f2f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052b-9710000000-b93562f16b5a93447a6f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0090000000-3c9d51b95e146c9f98a5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-2790000000-d7b050a5a96ddf960159 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9300000000-40d0fc7b0203b8ff8417 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0001458 |
|---|
| FooDB ID | FDB022636 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 388677 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 439597 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|