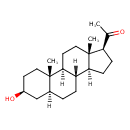
| (3beta,5alpha)-3-Hydroxypregnan-20-one | ChEBI |
| 3beta-Hydroxy-5alpha-pregnane-20-one | ChEBI |
| 5alpha-Pregnan-3beta-ol-20-one | ChEBI |
| Allopregnan-3beta-ol-20-one | ChEBI |
| Epiallopregnanolone | Kegg |
| 3beta-Hydroxy-5alpha-pregnan-20-one | Kegg |
| (3b,5a)-3-Hydroxypregnan-20-one | Generator |
| (3Β,5α)-3-hydroxypregnan-20-one | Generator |
| 3b-Hydroxy-5a-pregnane-20-one | Generator |
| 3Β-hydroxy-5α-pregnane-20-one | Generator |
| 5a-Pregnan-3b-ol-20-one | Generator |
| 5Α-pregnan-3β-ol-20-one | Generator |
| Allopregnan-3b-ol-20-one | Generator |
| Allopregnan-3β-ol-20-one | Generator |
| 3b-Hydroxy-5a-pregnan-20-one | Generator |
| 3Β-hydroxy-5α-pregnan-20-one | Generator |
| 3 Hydroxypregnan 20 one | MeSH |
| 3 alpha Hydroxy 5 alpha pregnan 20 one | MeSH |
| 3 alpha Hydroxy 5 beta pregnan 20 one | MeSH |
| 3 alpha, 5 beta Tetrahydroprogesterone | MeSH |
| 3 alpha, 5 beta-Tetrahydroprogesterone | MeSH |
| 3 alpha-Hydroxy-5 alpha-pregnan-20-one | MeSH |
| 3 alpha-Hydroxy-5 beta-pregnan-20-one | MeSH |
| 3-Hydroxypregnan-20-one | MeSH |
| 3beta Hydroxy 5alpha pregnan 20 one | MeSH |
| Allopregnan 3 beta ol 20 one | MeSH |
| Allopregnan-3 beta-ol-20-one | MeSH |
| Eltanolone | MeSH |
| Epipregnanolone | MeSH |
| Pregnan 3alpha ol 20 one | MeSH |
| Pregnan-3alpha-ol-20-one | MeSH |
| Pregnanolone | MeSH |
| Pregnanolone, (3alpha)-isomer | MeSH |
| Pregnanolone, (3alpha, 5beta, 17-alpha)-isomer | MeSH |
| Pregnanolone, (3alpha,5alpha)-isomer | MeSH |
| Pregnanolone, (3alpha,5beta)-isomer | MeSH |
| Pregnanolone, (3beta)-isomer | MeSH |
| Pregnanolone, (3beta, 5alpha)-isomer | MeSH |
| Pregnanolone, (3beta, 5alpha, 17alpha)-isomer | MeSH |
| Pregnanolone, (3beta, 5alpha, 8alpha, 17beta)-isomer | MeSH |
| Pregnanolone, (3beta, 5beta)-isomer | MeSH |
| Pregnanolone, (3beta, 5beta, 17alpha)-isomer | MeSH |
| Pregnanolone, (3beta, 5beta,14beta)-isomer | MeSH |
| Pregnanolone, (5alpha)-isomer | MeSH |
| Sepranolone | MeSH |
| alpha-Hydroxy-5 alpha-pregnan-20-one, 3 | MeSH |
| alpha-Hydroxy-5 beta-pregnan-20-one, 3 | MeSH |
| alpha-Pregnan-20-one, 3 alpha-hydroxy-5 | MeSH |
| beta-Ol-20-one, allopregnan-3 | MeSH |
| beta-Pregnan-20-one, 3 alpha-hydroxy-5 | MeSH |
| 3-deoxo-3b-Hydroxy-5a-dihydroprogesterone | HMDB |
| 3b-Allopregnanolone | HMDB |
| 3b-Hydroxy-5a,17b-pregnan-20-one | HMDB |
| 3b-Hydroxy-5a-tetrahydroprogesterone | HMDB |
| 5a-Dihydropregnenolone | HMDB |
| 5a-Pregnane-3b-ol-20-one | HMDB |
| Allopregnanolone | HMDB, MeSH |
| Isopregnanolone | HMDB |