| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 05:33:58 UTC |
|---|
| Update Date | 2016-11-09 01:21:16 UTC |
|---|
| Accession Number | CHEM035195 |
|---|
| Identification |
|---|
| Common Name | 2-Keto-3-deoxy-6-phosphogluconic acid |
|---|
| Class | Small Molecule |
|---|
| Description | The 5-phospho derivative of 2-dehydro-D-gluconic acid. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
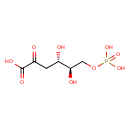
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-Dehydro-3-deoxy-6-phospho-D-gluconate | ChEBI | | 2-Dehydro-3-deoxy-D-gluconate 6-phosphate | ChEBI | | 2-Keto-3-deoxy-6-phosphogluconate | ChEBI | | 3-Deoxy-D-erythro-hex-2-ulosonic acid 6-phosphate | ChEBI | | 6-Phospho-2-dehydro-3-deoxy-D-gluconate | ChEBI | | KDPG Intermediate | ChEBI | | 2-Dehydro-3-deoxy-6-phospho-D-gluconic acid | Generator | | 2-Dehydro-3-deoxy-D-gluconic acid 6-phosphoric acid | Generator | | 3-Deoxy-D-erythro-hex-2-ulosonate 6-phosphate | Generator | | 3-Deoxy-D-erythro-hex-2-ulosonic acid 6-phosphoric acid | Generator | | 6-Phospho-2-dehydro-3-deoxy-D-gluconic acid | Generator | | KDPG Intermediic acid | Generator | | 2-dehydro-3-Deoxy-D-gluconate-6-phosphate | HMDB | | 2-keto-3-Deoxy-6-P-gluconate | HMDB | | 2-keto-3-Deoxy-6-phospho-gluconate | HMDB | | 2-keto-3-Deoxygluconate-6-P | HMDB | | 6-P-2-K-3-deo-Gluconate | HMDB | | 6-phospho-2-dehydro-3-Deoxygluconate | HMDB | | 6-phospho-2-keto-3-Deoxygluconate | HMDB |
|
|---|
| Chemical Formula | C6H11O9P |
|---|
| Average Molecular Mass | 258.120 g/mol |
|---|
| Monoisotopic Mass | 258.014 g/mol |
|---|
| CAS Registry Number | 27244-54-8 |
|---|
| IUPAC Name | (4S,5R)-4,5-dihydroxy-2-oxo-6-(phosphonooxy)hexanoic acid |
|---|
| Traditional Name | kdpg intermediate |
|---|
| SMILES | O[C@H](COP(O)(O)=O)[C@@H](O)CC(=O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C6H11O9P/c7-3(1-4(8)6(10)11)5(9)2-15-16(12,13)14/h3,5,7,9H,1-2H2,(H,10,11)(H2,12,13,14)/t3-,5+/m0/s1 |
|---|
| InChI Key | OVPRPPOVAXRCED-WVZVXSGGSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as medium-chain keto acids and derivatives. These are keto acids with a 6 to 12 carbon atoms long side chain. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Keto acids and derivatives |
|---|
| Sub Class | Medium-chain keto acids and derivatives |
|---|
| Direct Parent | Medium-chain keto acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Medium-chain keto acid
- Monoalkyl phosphate
- Alpha-keto acid
- Beta-hydroxy ketone
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Alkyl phosphate
- Alpha-hydroxy ketone
- Ketone
- 1,2-diol
- Secondary alcohol
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organooxygen compound
- Organic oxygen compound
- Carbonyl group
- Alcohol
- Organic oxide
- Hydrocarbon derivative
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0002-9310000000-b977edea41f10c14441f | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-01bc-6921400000-ca4d77ba878e92aaedf5 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-052f-1490000000-ea0f4bddcb2b9a63fa4f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01vy-6940000000-6658b367949c0aff033c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0076-9700000000-5dfc9ad3d3a20de41780 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a70-8290000000-46b63c8be3e78b565ecd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-9100000000-969c103f10917fe5078a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9000000000-6b6797b8569981c766a4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-06r7-6590000000-4bfcef169935d1cae20e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-9200000000-17589b3b796f6001e623 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0005-9000000000-cd26445e4e826ec3239a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-9040000000-51b487070c5625d5eab9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004j-9000000000-5c6f9eb036b780095d5a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9000000000-d484c197bb695cc95948 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0001376 |
|---|
| FooDB ID | FDB022588 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00019434 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | 2-KETO-3-DEOXY-6-P-GLUCONATE |
|---|
| METLIN ID | 6202 |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 2338483 |
|---|
| ChEBI ID | 15925 |
|---|
| PubChem Compound ID | 3080745 |
|---|
| Kegg Compound ID | C04442 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | ECMDB01376 |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|