| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 05:31:55 UTC |
|---|
| Update Date | 2016-11-09 01:21:15 UTC |
|---|
| Accession Number | CHEM035152 |
|---|
| Identification |
|---|
| Common Name | Diadenosine hexaphosphate |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
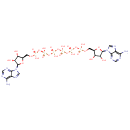
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Adenosine 5'-hexaphosphate 5'-ester with adenosine | ChEBI | | Adenosine-(5')-hexaphospho-(5')-adenosine | ChEBI | | Ap(6)a | ChEBI | | AP6a | ChEBI | | AppppppA | ChEBI | | Diadenosine 5',5''''-P1,P6-hexaphosphate | ChEBI | | P1,P6-Di(adenosine-5')hexaphosphate | ChEBI | | Adenosine 5'-hexaphosphoric acid 5'-ester with adenosine | Generator | | Diadenosine 5',5''''-P1,P6-hexaphosphoric acid | Generator | | P1,P6-Di(adenosine-5')hexaphosphoric acid | Generator | | Diadenosine hexaphosphoric acid | Generator | | Adenosine 5'-(heptahydrogen hexaphosphate) p->5'-ester with adenosine | HMDB | | Adenosine 5'-(heptahydrogen hexaphosphate)5'-5'-ester with adenosine | HMDB | | P1,P6-Bis(5'-adenosyl)hexaphosphoric acid | HMDB | | p(1),p(6)-Bis(5'-adenosyl)hexaphosphoric acid | HMDB | | Diadenosine hexaphosphate | ChEBI |
|
|---|
| Chemical Formula | C20H30N10O25P6 |
|---|
| Average Molecular Mass | 996.347 g/mol |
|---|
| Monoisotopic Mass | 995.981 g/mol |
|---|
| CAS Registry Number | 56983-23-4 |
|---|
| IUPAC Name | {[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy}({[({[({[({[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy](hydroxy)phosphoryl}oxy)(hydroxy)phosphoryl]oxy}(hydroxy)phosphoryl)oxy](hydroxy)phosphoryl}oxy)phosphinic acid |
|---|
| Traditional Name | AppppppA |
|---|
| SMILES | NC1=C2N=CN([C@@H]3O[C@H](COP(O)(=O)OP(O)(=O)OP(O)(=O)OP(O)(=O)OP(O)(=O)OP(O)(=O)OC[C@H]4O[C@H]([C@H](O)[C@@H]4O)N4C=NC5=C(N)N=CN=C45)[C@@H](O)[C@H]3O)C2=NC=N1 |
|---|
| InChI Identifier | InChI=1S/C20H30N10O25P6/c21-15-9-17(25-3-23-15)29(5-27-9)19-13(33)11(31)7(49-19)1-47-56(35,36)51-58(39,40)53-60(43,44)55-61(45,46)54-59(41,42)52-57(37,38)48-2-8-12(32)14(34)20(50-8)30-6-28-10-16(22)24-4-26-18(10)30/h3-8,11-14,19-20,31-34H,1-2H2,(H,35,36)(H,37,38)(H,39,40)(H,41,42)(H,43,44)(H,45,46)(H2,21,23,25)(H2,22,24,26)/t7-,8-,11-,12-,13-,14-,19-,20-/m1/s1 |
|---|
| InChI Key | PZCFFCOJNXGTIM-XPWFQUROSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as (5'->5')-dinucleotides. These are dinucleotides where the two bases are connected via a (5'->5')-phosphodiester linkage. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Nucleosides, nucleotides, and analogues |
|---|
| Class | (5'->5')-dinucleotides |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | (5'->5')-dinucleotides |
|---|
| Alternative Parents | |
|---|
| Substituents | - (5'->5')-dinucleotide
- Purine ribonucleoside polyphosphate
- Purine nucleotide sugar
- Purine ribonucleoside monophosphate
- Pentose phosphate
- Pentose-5-phosphate
- Glycosyl compound
- N-glycosyl compound
- 6-aminopurine
- Monosaccharide phosphate
- Purine
- Imidazopyrimidine
- Monoalkyl phosphate
- Aminopyrimidine
- Alkyl phosphate
- Monosaccharide
- N-substituted imidazole
- Organic phosphoric acid derivative
- Pyrimidine
- Phosphoric acid ester
- Imidolactam
- Tetrahydrofuran
- Imidazole
- Azole
- Heteroaromatic compound
- Secondary alcohol
- Azacycle
- Oxacycle
- Organoheterocyclic compound
- Hydrocarbon derivative
- Alcohol
- Organonitrogen compound
- Organooxygen compound
- Organic oxygen compound
- Organic nitrogen compound
- Organopnictogen compound
- Organic oxide
- Amine
- Primary amine
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0910110002-59d115c90a4481a6b007 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0900000000-d3bcef479d59e691d5b8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-0900000000-8dec47b8f02e8a6ac5a7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000x-0700000209-de23da8b809775f36d57 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-0900000200-eec87d1063e1b19d703a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-003r-1902401000-c06f9d4097503496c4b3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0000000009-1da203db6bf6303096a0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-0100001329-9afe5ff0f93ff13bf2a8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000b-0103149204-ccb4974c1451a6de78f3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0100000009-e59c713225edd8e0460d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0300000039-0a05007bbbf9147ab5f0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-0900000110-66251e85696bf2211dd4 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0001282 |
|---|
| FooDB ID | FDB022533 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | B6P |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 110267 |
|---|
| ChEBI ID | 63689 |
|---|
| PubChem Compound ID | 123694 |
|---|
| Kegg Compound ID | C20190 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | ECMDB24064 |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Sawai, Hiroaki; Inaba, Toshiko; Hirano, Atsushi; Wakai, Hiromichi; Shimazu, Masamitsu. Magnesium(II) ion-mediated conversion of mono- and oligonucleotides to 5'-polyphosphates in aqueous solution. Tetrahedron Letters (1993), 34(30), 4801-4. | | 2. Jankowski J, Potthoff W, van der Giet M, Tepel M, Zidek W, Schluter H: High-performance liquid chromatographic assay of the diadenosine polyphosphates in human platelets. Anal Biochem. 1999 Apr 10;269(1):72-8. | | 3. Flores NA, Stavrou BM, Sheridan DJ: The effects of diadenosine polyphosphates on the cardiovascular system. Cardiovasc Res. 1999 Apr;42(1):15-26. | | 4. Jankowski J, Tepel M, van der Giet M, Tente IM, Henning L, Junker R, Zidek W, Schluter H: Identification and characterization of P(1), P(7)-Di(adenosine-5')-heptaphosphate from human platelets. J Biol Chem. 1999 Aug 20;274(34):23926-31. | | 5. Luo J, Jankowski J, Tepel M, von Der Giet M, Zidek W, Schluter H: Identification of diadenosine hexaphosphate in human erythrocytes. Hypertension. 1999 Oct;34(4 Pt 2):872-5. | | 6. Yang X, Safrany ST, Shears SB: Site-directed mutagenesis of diphosphoinositol polyphosphate phosphohydrolase, a dual specificity NUDT enzyme that attacks diadenosine polyphosphates and diphosphoinositol polyphosphates. J Biol Chem. 1999 Dec 10;274(50):35434-40. | | 7. Conant AR, Fisher MJ, McLennan AG, Simpson AW: Diadenosine polyphosphates are largely ineffective as agonists at natively expressed P2Y(1) and P2Y(2) receptors on cultured human saphenous vein endothelial cells. J Vasc Res. 2000 Nov-Dec;37(6):548-55. | | 8. Hollah P, Hausberg M, Kosch M, Barenbrock M, Letzel M, Schlatter E, Rahn KH: A novel assay for determination of diadenosine polyphosphates in human platelets: studies in normotensive subjects and in patients with essential hypertension. J Hypertens. 2001 Feb;19(2):237-45. | | 9. Patel K, Barnes A, Camacho J, Paterson C, Boughtflower R, Cousens D, Marshall F: Activity of diadenosine polyphosphates at P2Y receptors stably expressed in 1321N1 cells. Eur J Pharmacol. 2001 Nov 2;430(2-3):203-10. | | 10. Steinmetz M, Janssen AK, Pelster F, Rahn KH, Schlatter E: Vasoactivity of diadenosine polyphosphates in human small mesenteric resistance arteries. J Pharmacol Exp Ther. 2002 Aug;302(2):787-94. | | 11. Miras-Portugal MT, Pintor J, Gualix J: Ca2+ signalling in brain synaptosomes activated by dinucleotides. J Membr Biol. 2003 Jul 1;194(1):1-10. | | 12. Luo J, Jankowski V, Gungar N, Neumann J, Schmitz W, Zidek W, Schluter H, Jankowski J: Endogenous diadenosine tetraphosphate, diadenosine pentaphosphate, and diadenosine hexaphosphate in human myocardial tissue. Hypertension. 2004 May;43(5):1055-9. Epub 2004 Apr 5. | | 13. Stavrou BM: Diadenosine polyphosphates: postulated mechanisms mediating the cardiac effects. Curr Med Chem Cardiovasc Hematol Agents. 2003 Jun;1(2):151-69. | | 14. Davies G, MacAllister RJ, Bogle RG, Vallance P: Effect of diadenosine phosphates on human umbilical vessels: novel platelet-derived vasoconstrictors. Br J Clin Pharmacol. 1995 Aug;40(2):170-2. | | 15. Tepel M, Lowe S, Nofer JR, Assmann G, Schluter H, Zidek W: Diadenosine polyphosphates regulate cytosolic calcium in human fibroblast cells by interaction with P2x purinoceptors coupled to phospholipase C. Biochim Biophys Acta. 1996 Jun 13;1312(2):145-50. | | 16. Vahlensieck U, Boknik P, Knapp J, Linck B, Muller FU, Neumann J, Herzig S, Schluter H, Zidek W, Deng MC, Scheld HH, Schmitz W: Negative chronotropic and inotropic effects exerted by diadenosine hexaphosphate (AP6A) via A1-adenosine receptors. Br J Pharmacol. 1996 Nov;119(5):835-44. | | 17. Gasmi L, McLennan AG, Edwards SW: Neutrophil apoptosis is delayed by the diadenosine polyphosphates, Ap5A and Ap6A: synergism with granulocyte-macrophage colony-stimulating factor. Br J Haematol. 1996 Dec;95(4):637-9. | | 18. Ogilvie A, Blasius R, Schulze-Lohoff E, Sterzel RB: Adenine dinucleotides: a novel class of signalling molecules. J Auton Pharmacol. 1996 Dec;16(6):325-8. | | 19. Jankowski J, Schluter H, Tepel M, Spieker C, Zidek W: Effect of diadenosine polyphosphates on Ca2+ ATPase activity. J Mol Med (Berl). 1997 Sep;75(9):674-7. | | 20. Stachon A, Stegemann H, Hohage H, Rahn KH, Schlatter E: Effects of diadenosine polyphosphates on the intracellular Ca2+ concentration in endothelial cells. Cell Physiol Biochem. 1998;8(4):175-84. | | 21. Jankowski J, Jankowski V, Laufer U, van der Giet M, Henning L, Tepel M, Zidek W, Schluter H: Identification and quantification of diadenosine polyphosphate concentrations in human plasma. Arterioscler Thromb Vasc Biol. 2003 Jul 1;23(7):1231-8. Epub 2003 May 8. | | 22. Pintor J, Carracedo G, Alonso MC, Bautista A, Peral A: Presence of diadenosine polyphosphates in human tears. Pflugers Arch. 2002 Jan;443(3):432-6. Epub 2001 Aug 23. | | 23. Kisselev LL, Justesen J, Wolfson AD, Frolova LY: Diadenosine oligophosphates (Ap(n)A), a novel class of signalling molecules? FEBS Lett. 1998 May 8;427(2):157-63. | | 24. Pintor J, King BF, Miras-Portugal MT, Burnstock G: Selectivity and activity of adenine dinucleotides at recombinant P2X2 and P2Y1 purinoceptors. Br J Pharmacol. 1996 Nov;119(5):1006-12. | | 25. Baker MD, Holloway DE, Swaminathan GJ, Acharya KR: Crystal structures of eosinophil-derived neurotoxin (EDN) in complex with the inhibitors 5'-ATP, Ap3A, Ap4A, and Ap5A. Biochemistry. 2006 Jan 17;45(2):416-26. | | 26. https://www.ncbi.nlm.nih.gov/pubmed/?term=10385004 | | 27. https://www.ncbi.nlm.nih.gov/pubmed/?term=10419486 | | 28. https://www.ncbi.nlm.nih.gov/pubmed/?term=10523376 | | 29. https://www.ncbi.nlm.nih.gov/pubmed/?term=11327631 | | 30. https://www.ncbi.nlm.nih.gov/pubmed/?term=12121577 | | 31. https://www.ncbi.nlm.nih.gov/pubmed/?term=14502438 | | 32. https://www.ncbi.nlm.nih.gov/pubmed/?term=15066958 | | 33. https://www.ncbi.nlm.nih.gov/pubmed/?term=1632493 | | 34. https://www.ncbi.nlm.nih.gov/pubmed/?term=17824959 | | 35. https://www.ncbi.nlm.nih.gov/pubmed/?term=20190246 | | 36. https://www.ncbi.nlm.nih.gov/pubmed/?term=20807533 | | 37. https://www.ncbi.nlm.nih.gov/pubmed/?term=7769127 | | 38. https://www.ncbi.nlm.nih.gov/pubmed/?term=8114917 | | 39. https://www.ncbi.nlm.nih.gov/pubmed/?term=8566144 |
|
|---|