| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 05:31:49 UTC |
|---|
| Update Date | 2016-11-09 01:21:15 UTC |
|---|
| Accession Number | CHEM035150 |
|---|
| Identification |
|---|
| Common Name | Presqualene diphosphate |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
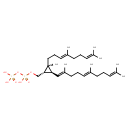
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| [(1S,2S,3S)-2-[(e)-4,8-Dimethylnona-3,7-dienyl]-2-methyl-3-[(2E,6E)-2,6,10-trimethylundeca-1,5,9-trienyl]cyclopropan-1-yl]methyl diphosphate | ChEBI | | [(1S,2S,3S)-2-[(e)-4,8-Dimethylnona-3,7-dienyl]-2-methyl-3-[(2E,6E)-2,6,10-trimethylundeca-1,5,9-trienyl]cyclopropan-1-yl]methyl diphosphoric acid | Generator | | Presqualene diphosphoric acid | Generator | | [(1R,2R,3R)-2-[(e)-4,8-Dimethylnona-3,7-dienyl]-2-methyl-3-[(2E,6E)-2,6,10-trimethylundeca-1,5,9-trienyl]cyclopropan-1-yl]methyl diphosphate | HMDB | | [(1R,2R,3R)-2-[(e)-4,8-Dimethylnona-3,7-dienyl]-2-methyl-3-[(2E,6E)-2,6,10-trimethylundeca-1,5,9-trienyl]cyclopropan-1-yl]methyl diphosphoric acid | HMDB | | (1alpha,2beta(e),3beta(1E,5E))-Diphosphoric acid mono((2-(4,8-dimethyl-3,7-nonadienyl)-2-methyl-3-(2,6,10-trimethyl-1,5,9-undecatrienyl)cyclopropyl)methyl) ester | HMDB | | Presqualene pyrophosphate | HMDB | | Presqualene diphosphate | HMDB |
|
|---|
| Chemical Formula | C30H52O7P2 |
|---|
| Average Molecular Mass | 586.677 g/mol |
|---|
| Monoisotopic Mass | 586.319 g/mol |
|---|
| CAS Registry Number | 29849-75-0 |
|---|
| IUPAC Name | [({[(1S,2S,3S)-2-[(3E)-4,8-dimethylnona-3,7-dien-1-yl]-2-methyl-3-[(1E,5E)-2,6,10-trimethylundeca-1,5,9-trien-1-yl]cyclopropyl]methoxy}(hydroxy)phosphoryl)oxy]phosphonic acid |
|---|
| Traditional Name | presqualene diphosphate |
|---|
| SMILES | CC(C)=CCC\C(C)=C\CC\C(C)=C\[C@@]1([H])[C@]([H])(COP(O)(=O)OP(O)(O)=O)[C@@]1(C)CC\C=C(/C)CCC=C(C)C |
|---|
| InChI Identifier | InChI=1S/C30H52O7P2/c1-23(2)13-9-15-25(5)17-11-18-27(7)21-28-29(22-36-39(34,35)37-38(31,32)33)30(28,8)20-12-19-26(6)16-10-14-24(3)4/h13-14,17,19,21,28-29H,9-12,15-16,18,20,22H2,1-8H3,(H,34,35)(H2,31,32,33)/b25-17+,26-19+,27-21+/t28-,29-,30-/m0/s1 |
|---|
| InChI Key | ATZKAUGGNMSCCY-VYCBRMPGSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as triterpenoids. These are terpene molecules containing six isoprene units. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Triterpenoids |
|---|
| Direct Parent | Triterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Triterpenoid
- Organic pyrophosphate
- Monoalkyl phosphate
- Alkyl phosphate
- Phosphoric acid ester
- Organic phosphoric acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0gia-4200490000-9a3a83875f6f2ef2cddb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4r-0200690000-b880904a199b28acdc23 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-2352900000-82d62bcceb1656998b10 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a6r-3366920000-574a4a9be60ca4c8d765 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0500090000-557817e75ec0eed28672 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-056r-9800020000-6bca51e71e8dd4501523 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9000000000-0fe8c0f845a21c70b30c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0100090000-5247a3c4a85015b072da | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-002r-2200090000-09d9e50734c089804e41 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9100000000-7dab7bce5c804b18ac2b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0232590000-730292814c7f9261de9b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014j-3402890000-7a23c1768de1e2a2244f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-9317500000-d5d421cf835631935344 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0001278 |
|---|
| FooDB ID | FDB022531 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | CPD-465 |
|---|
| METLIN ID | 6132 |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 4444207 |
|---|
| ChEBI ID | 15442 |
|---|
| PubChem Compound ID | 5280592 |
|---|
| Kegg Compound ID | C03428 |
|---|
| YMDB ID | YMDB00843 |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|