| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 05:30:54 UTC |
|---|
| Update Date | 2016-11-09 01:21:15 UTC |
|---|
| Accession Number | CHEM035132 |
|---|
| Identification |
|---|
| Common Name | 5-Aminoimidazole ribonucleotide |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
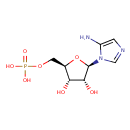
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-(5'-Phosphoribosyl)-5-aminoimidazole | ChEBI | | 1-(5-Phospho-D-ribosyl)-5-aminoimidazole | ChEBI | | 5'-Phosphoribosyl-5-aminoimidazole | ChEBI | | 5-Amino-1-beta-D-ribofuranosyl-5'-(dihydrogen phosphate)-imidazole | ChEBI | | 5-Amino-1-ribofuranosylimidazole 5'-phosphate | ChEBI | | AIR | ChEBI | | Aminoimidazole ribonucleotide | ChEBI | | Aminoimidazole ribotide | ChEBI | | Phosphoribosylaminoimidazole | ChEBI | | 5-Amino-1-(5-phospho-D-ribosyl)imidazole | Kegg | | 5-Amino-1-(5-phospho-beta-D-ribosyl)imidazole | Kegg | | 5-Amino-1-b-D-ribofuranosyl-5'-(dihydrogen phosphate)-imidazole | Generator | | 5-Amino-1-b-D-ribofuranosyl-5'-(dihydrogen phosphoric acid)-imidazole | Generator | | 5-Amino-1-beta-D-ribofuranosyl-5'-(dihydrogen phosphoric acid)-imidazole | Generator | | 5-Amino-1-β-D-ribofuranosyl-5'-(dihydrogen phosphate)-imidazole | Generator | | 5-Amino-1-β-D-ribofuranosyl-5'-(dihydrogen phosphoric acid)-imidazole | Generator | | 5-Amino-1-ribofuranosylimidazole 5'-phosphoric acid | Generator | | 5-Amino-1-(5-phospho-b-D-ribosyl)imidazole | Generator | | 5-Amino-1-(5-phospho-β-D-ribosyl)imidazole | Generator | | 5-Aminoimidazole ribotide | HMDB | | Aminoimidazole ribotide, (beta-D-ribofuranosyl)-isomer | HMDB | | Aminoimidazole ribotide, (alpha-D-ribofuranosyl)-isomer | HMDB | | Aminoimidazole ribotide, phosphonoribofuranosyl-isomer | HMDB |
|
|---|
| Chemical Formula | C8H14N3O7P |
|---|
| Average Molecular Mass | 295.186 g/mol |
|---|
| Monoisotopic Mass | 295.057 g/mol |
|---|
| CAS Registry Number | 25635-88-5 |
|---|
| IUPAC Name | {[(2R,3S,4R,5R)-5-(5-amino-1H-imidazol-1-yl)-3,4-dihydroxyoxolan-2-yl]methoxy}phosphonic acid |
|---|
| Traditional Name | 5-aminoimidazole ribotide |
|---|
| SMILES | NC1=CN=CN1[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](O)[C@H]1O |
|---|
| InChI Identifier | InChI=1S/C8H14N3O7P/c9-5-1-10-3-11(5)8-7(13)6(12)4(18-8)2-17-19(14,15)16/h1,3-4,6-8,12-13H,2,9H2,(H2,14,15,16)/t4-,6-,7-,8-/m1/s1 |
|---|
| InChI Key | PDACUKOKVHBVHJ-XVFCMESISA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pentose phosphates. These are carbohydrate derivatives containing a pentose substituted by one or more phosphate groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Pentose phosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pentose phosphate
- Pentose-5-phosphate
- Imidazole ribonucleoside
- Glycosyl compound
- N-glycosyl compound
- Monosaccharide phosphate
- Monoalkyl phosphate
- Aminoimidazole
- Alkyl phosphate
- Phosphoric acid ester
- N-substituted imidazole
- Organic phosphoric acid derivative
- Heteroaromatic compound
- Azole
- Tetrahydrofuran
- Imidazole
- 1,2-diol
- Secondary alcohol
- Oxacycle
- Organoheterocyclic compound
- Azacycle
- Organic oxide
- Primary amine
- Organic nitrogen compound
- Alcohol
- Hydrocarbon derivative
- Amine
- Organopnictogen compound
- Organonitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0002-9310000000-83cf50bc3f675ec17ccb | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-01vk-9314000000-ed8269ba053b2e3a4fc4 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-9130000000-143cf7dfaf8cc77d6280 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-9000000000-26b4d68163a88586005b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-9000000000-87449f0e16a1690c4873 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0036-9160000000-50e60dc03e3bb2050636 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0059-9000000000-797c3e0faf96fe4330aa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9000000000-9e3a71c432cedbcd3892 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-2490000000-7dd58fcac26f75e7c46f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-9000000000-6dd5f8e7028cd1ab65d2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-9000000000-7542d4d80a10b03631f0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-002f-9080000000-e3666a5af349af6427f9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-9000000000-2dd173dfe9a30642b89d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9000000000-7c5243a801196e080ddb | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0001235 |
|---|
| FooDB ID | FDB022504 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00019655 |
|---|
| BiGG ID | 41727 |
|---|
| BioCyc ID | 5-PHOSPHORIBOSYL-5-AMINOIMIDAZOLE |
|---|
| METLIN ID | 6097 |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | 5-Aminoimidazole_ribotide |
|---|
| Chemspider ID | 141854 |
|---|
| ChEBI ID | 138560 |
|---|
| PubChem Compound ID | 161500 |
|---|
| Kegg Compound ID | C03373 |
|---|
| YMDB ID | YMDB00065 |
|---|
| ECMDB ID | ECMDB01235 |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Groziak M P; Bhat B; Leonard N J Nonenzymatic synthesis of 5-aminoimidazole ribonucleoside and recognition of its facile rearrangement. Proceedings of the National Academy of Sciences of the United States of America (1988), 85(19), 7174-6. | | 2. Vetvik H, Grewal HM, Haugen IL, Ahren C, Haneberg B: Mucosal antibodies can be measured in air-dried samples of saliva and feces. J Immunol Methods. 1998 Jun 1;215(1-2):163-72. | | 3. Ogata M, Michitsuji H, Fujiki Y: Estimating amounts of toluene inhaled by workers with protective mask using biological indicators of toluene. Toxicol Lett. 1999 Sep 5;108(2-3):233-9. | | 4. Chang HK, Weber ME, King M: Mucus transport by high-frequency nonsymmetrical oscillatory airflow. J Appl Physiol (1985). 1988 Sep;65(3):1203-9. | | 5. Lawhorn BG, Mehl RA, Begley TP: Biosynthesis of the thiamin pyrimidine: the reconstitution of a remarkable rearrangement reaction. Org Biomol Chem. 2004 Sep 7;2(17):2538-46. Epub 2004 Aug 11. | | 6. https://www.ncbi.nlm.nih.gov/pubmed/?term=21135073 | | 7. https://www.ncbi.nlm.nih.gov/pubmed/?term=21548610 | | 8. https://www.ncbi.nlm.nih.gov/pubmed/?term=24525042 | | 9. https://www.ncbi.nlm.nih.gov/pubmed/?term=25372694 | | 10. https://www.ncbi.nlm.nih.gov/pubmed/?term=26100042 | | 11. https://www.ncbi.nlm.nih.gov/pubmed/?term=26237670 | | 12. https://www.ncbi.nlm.nih.gov/pubmed/?term=26524231 | | 13. https://www.ncbi.nlm.nih.gov/pubmed/?term=27264098 |
|
|---|