| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 05:29:53 UTC |
|---|
| Update Date | 2016-11-09 01:21:15 UTC |
|---|
| Accession Number | CHEM035110 |
|---|
| Identification |
|---|
| Common Name | 2(N)-Methyl-norsalsolinol |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
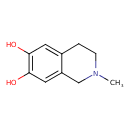
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1,2,3,4-tetrahydro-2-Methyl-6,7-isoquinolinediol | HMDB | | 2-Methyl-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline | HMDB, MeSH | | 2-Methyl-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline hydrobromide | MeSH, HMDB | | NMNSAL | MeSH, HMDB | | 2-MDTIQ | MeSH, HMDB | | 2-Methyl-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline hydrochloride | MeSH, HMDB | | N-Methylnorsalsolinol | MeSH, HMDB |
|
|---|
| Chemical Formula | C10H13NO2 |
|---|
| Average Molecular Mass | 179.216 g/mol |
|---|
| Monoisotopic Mass | 179.095 g/mol |
|---|
| CAS Registry Number | 63937-92-8 |
|---|
| IUPAC Name | 2-methyl-1,2,3,4-tetrahydroisoquinoline-6,7-diol |
|---|
| Traditional Name | 2(N)-methyl-norsalsolinol |
|---|
| SMILES | CN1CCC2=CC(O)=C(O)C=C2C1 |
|---|
| InChI Identifier | InChI=1S/C10H13NO2/c1-11-3-2-7-4-9(12)10(13)5-8(7)6-11/h4-5,12-13H,2-3,6H2,1H3 |
|---|
| InChI Key | WETXYFNVBDLWIW-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as tetrahydroisoquinolines. These are tetrahydrogenated isoquinoline derivatives. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Tetrahydroisoquinolines |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Tetrahydroisoquinolines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Tetrahydroisoquinoline
- 1-hydroxy-2-unsubstituted benzenoid
- Aralkylamine
- Benzenoid
- Tertiary aliphatic amine
- Tertiary amine
- Azacycle
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Amine
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0h09-0900000000-bf4ec1062ac2b839282e | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-05fr-1193000000-f8ec40086f347c196a77 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0900000000-2d6d43a51cbdcaacb791 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0f89-0900000000-9da5452488647aecc4ac | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0079-5900000000-b48adad337bbc3b4c3f2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0900000000-59c2c966e5bb27fa34a4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-0900000000-4d8538c59706a2953c8b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-074l-2900000000-f74a421baa4902e5b4f8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0900000000-e9e4d0ba83478e578c42 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-0900000000-cab123cec057104fdf65 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00dr-1900000000-88f6a4deeb5a022ef56a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0900000000-6326446584297a254a6a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-0900000000-d5a1eb90c086c0424943 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05mx-8900000000-66d4fc031f9937d4b4dd | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0001189 |
|---|
| FooDB ID | FDB022477 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 34632 |
|---|
| ChEBI ID | 767276 |
|---|
| PubChem Compound ID | 37764 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Moser A, Scholz J, Nobbe F, Vieregge P, Bohme V, Bamberg H: Presence of N-methyl-norsalsolinol in the CSF: correlations with dopamine metabolites of patients with Parkinson's disease. J Neurol Sci. 1995 Aug;131(2):183-9. | | 2. Thumen A, Behnecke A, Qadri F, Bauml E, Thumen A, Behnecke CA, Qadri F, Bauml E, Moser A: N-methyl-norsalsolinol, a putative dopaminergic neurotoxin, passes through the blood-brain barrier in vivo. Neuroreport. 2002 Jan 21;13(1):25-8. | | 3. Moser A, Thumen A, Qadri F: Modulation of striatal serotonin and opioid receptor mRNA expression following systemic N-methyl-norsalsolinol administration. J Neurol Sci. 2003 Dec 15;216(1):109-12. |
|
|---|