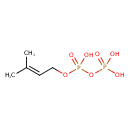
| 2-Isopentenyl diphosphate | ChEBI |
| 3,3-Dimethylallyl pyrophosphate | ChEBI |
| 3-Methylbut-2-enyl phosphono hydrogen phosphate | ChEBI |
| delta-Prenyl diphosphate | ChEBI |
| delta2-Isopentenyl diphosphate | ChEBI |
| Dimethylallyl diphosphate | ChEBI |
| Dimethylallyl pyrophosphate | ChEBI |
| DMAPP | ChEBI |
| Monoprenyl diphosphate | ChEBI |
| Prenol pyrophosphate | ChEBI |
| Prenyl diphosphate | Kegg |
| 2-Isopentenyl diphosphoric acid | Generator |
| 3,3-Dimethylallyl pyrophosphoric acid | Generator |
| 3-Methylbut-2-enyl phosphono hydrogen phosphoric acid | Generator |
| delta-Prenyl diphosphoric acid | Generator |
| Δ-prenyl diphosphate | Generator |
| Δ-prenyl diphosphoric acid | Generator |
| delta2-Isopentenyl diphosphoric acid | Generator |
| Δ2-isopentenyl diphosphate | Generator |
| Δ2-isopentenyl diphosphoric acid | Generator |
| Dimethylallyl diphosphoric acid | Generator |
| Dimethylallyl pyrophosphoric acid | Generator |
| Monoprenyl diphosphoric acid | Generator |
| Prenol pyrophosphoric acid | Generator |
| Prenyl diphosphoric acid | Generator |
| Dimethylallylpyrophosphoric acid | Generator |
| 1,1-Dimethyl-4-phenylpiperazinium iodide | HMDB |
| 3-Methyl-2-buten-1-ol pyrophosphate | HMDB |
| 3-Methyl-2-buten-1-ol trihydrogen pyrophosphate | HMDB |
| 3-Methyl-2-butenyl pyrophosphate | HMDB |
| 3-Methylbut-2-enyl pyrophosphate | HMDB |
| Delta2-Isopentenyl-diphosphate | HMDB |
| Dimethylallyl-diphosphate | HMDB |
| Dimethylallyl-PP | HMDB |
| Dimethylallyl-ppi | HMDB |
| Dimethylallyl-pyrophosphate | HMDB |
| Diphosphoric acid mono(3-methyl-2-butenyl) ester | HMDB |
| DMPP | HMDB |
| IPE | HMDB |
| Prenyl-diphosphate | HMDB |
| 3,3-Dimethylallyl pyrophosphate, (14)C-labeled | HMDB |
| DMADP CPD | HMDB |
| 3-Methyl-2-butenyl trihydrogen diphosphate | HMDB |
| Dimethylallylpyrophosphate | HMDB |
| gamma,gamma-Dimethylallyl pyrophosphate | HMDB |
| γ,γ-Dimethylallyl pyrophosphate | HMDB |