| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 05:28:19 UTC |
|---|
| Update Date | 2016-11-09 01:21:14 UTC |
|---|
| Accession Number | CHEM035076 |
|---|
| Identification |
|---|
| Common Name | 3-Aminopropionaldehyde |
|---|
| Class | Small Molecule |
|---|
| Description | 3-Aminopropionaldehyde, also known as 3-aminopropanal, belongs to the class of organic compounds known as alpha-hydrogen aldehydes. These are aldehydes with the general formula HC(H)(R)C(=O)H, where R is an organyl group. 3-Aminopropionaldehyde is possibly soluble (in water) and a very strong basic compound (based on its pKa). 3-Aminopropionaldehyde exists in all living species, ranging from bacteria to humans. 3-Aminopropionaldehyde participates in a number of enzymatic reactions, within cattle. In particular, 3-Aminopropionaldehyde can be converted into beta-alanine through the action of the enzyme aldehyde dehydrogenase, mitochondrial. In addition, 3-Aminopropionaldehyde can be biosynthesized from 1,3-diaminopropane through its interaction with the enzyme membrane primary amine oxidase. In cattle, 3-aminopropionaldehyde is involved in the metabolic pathway called the beta-alanine metabolism pathway. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
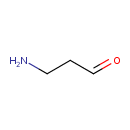
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| beta-Aminopropion aldehyde | ChEBI | | b-Aminopropion aldehyde | Generator | | Β-aminopropion aldehyde | Generator | | 3-Aminopropanal | HMDB |
|
|---|
| Chemical Formula | C3H7NO |
|---|
| Average Molecular Mass | 73.094 g/mol |
|---|
| Monoisotopic Mass | 73.053 g/mol |
|---|
| CAS Registry Number | 352-92-1 |
|---|
| IUPAC Name | 3-aminopropanal |
|---|
| Traditional Name | 3-aminopropanal |
|---|
| SMILES | NCCC=O |
|---|
| InChI Identifier | InChI=1S/C3H7NO/c4-2-1-3-5/h3H,1-2,4H2 |
|---|
| InChI Key | PCXDJQZLDDHMGX-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as alpha-hydrogen aldehydes. These are aldehydes with the general formula HC(H)(R)C(=O)H, where R is an organyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbonyl compounds |
|---|
| Direct Parent | Alpha-hydrogen aldehydes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-hydrogen aldehyde
- Organic nitrogen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Primary amine
- Organonitrogen compound
- Primary aliphatic amine
- Amine
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-001i-9000000000-13fb77ed3773ab13b10f | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0ab9-9000000000-8e068317c3b7ab851129 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-9000000000-e78722b5912332b96551 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9000000000-63fc6b8083111d21b561 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-9000000000-bfbb5311d3b0abfbe795 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-9000000000-68e71bb6c15dff78a8c2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9000000000-8780aca699fa02169cf4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-9000000000-796247bade5fd5732593 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-9000000000-1ec800454111a0c64d46 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9000000000-a2ff71f6703a0a4daa57 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-9000000000-bc974757d0839ce6b57c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-9000000000-04ae037d22ea245042cf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-9000000000-be4ae43b8bb9483fb624 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0001106 |
|---|
| FooDB ID | FDB030407 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | 46232 |
|---|
| BioCyc ID | CPD-6082 |
|---|
| METLIN ID | 6007 |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 74 |
|---|
| ChEBI ID | 18090 |
|---|
| PubChem Compound ID | 75 |
|---|
| Kegg Compound ID | C05665 |
|---|
| YMDB ID | YMDB00048 |
|---|
| ECMDB ID | ECMDB01106 |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Qian L, Zhao A, Zhang Y, Chen T, Zeisel SH, Jia W, Cai W: Metabolomic Approaches to Explore Chemical Diversity of Human Breast-Milk, Formula Milk and Bovine Milk. Int J Mol Sci. 2016 Dec 17;17(12). pii: ijms17122128. doi: 10.3390/ijms17122128. | | 2. Bott, Kaspar. Amidomethylation of trichloroethylene and acetylene: syntheses of N-protected a-chloro-b-alanines and b-aminopropanals. Journal of the Chemical Society [Section] D: Chemical Communications (1969), (22), 1304. | | 3. Ivanova S, Batliwalla F, Mocco J, Kiss S, Huang J, Mack W, Coon A, Eaton JW, Al-Abed Y, Gregersen PK, Shohami E, Connolly ES Jr, Tracey KJ: Neuroprotection in cerebral ischemia by neutralization of 3-aminopropanal. Proc Natl Acad Sci U S A. 2002 Apr 16;99(8):5579-84. Epub 2002 Apr 9. | | 4. Yu Z, Li W, Hillman J, Brunk UT: Human neuroblastoma (SH-SY5Y) cells are highly sensitive to the lysosomotropic aldehyde 3-aminopropanal. Brain Res. 2004 Aug 6;1016(2):163-9. |
|
|---|