| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 05:26:04 UTC |
|---|
| Update Date | 2016-11-09 01:21:14 UTC |
|---|
| Accession Number | CHEM035034 |
|---|
| Identification |
|---|
| Common Name | Malyl-CoA |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
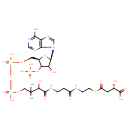
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Malyl-coenzyme A | MeSH | | (3S)-3-Carboxy-3-hydroxypropanoyl-CoA | HMDB | | (3S)-3-Carboxy-3-hydroxypropanoyl-coenzyme A | HMDB | | (3S)-3-Carboxy-3-hydroxypropionyl-CoA | HMDB | | (3S)-3-Carboxy-3-hydroxypropionyl-coenzyme A | HMDB | | L-Malyl-CoA | HMDB | | L-Malyl-coenzyme A | HMDB | | S-(3-Carboxy-3-hydroxypropanoate | HMDB | | S-(3-Carboxy-3-hydroxypropanoate) CoA | HMDB | | S-(3-Carboxy-3-hydroxypropanoate) coenzyme A | HMDB | | S-(3-Carboxy-3-hydroxypropanoic acid | HMDB |
|
|---|
| Chemical Formula | C25H40N7O20P3S |
|---|
| Average Molecular Mass | 883.606 g/mol |
|---|
| Monoisotopic Mass | 883.126 g/mol |
|---|
| CAS Registry Number | 2043-93-8 |
|---|
| IUPAC Name | (2S)-4-{[2-(3-{3-[({[({[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-3-(phosphonooxy)oxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy](hydroxy)phosphoryl}oxy)methyl]-2-hydroxy-3-methylbutanamido}propanamido)ethyl]sulfanyl}-2-hydroxy-4-oxobutanoic acid |
|---|
| Traditional Name | (2S)-4-({2-[3-(3-{[({[(2R,3S,4R,5R)-5-(6-aminopurin-9-yl)-4-hydroxy-3-(phosphonooxy)oxolan-2-yl]methoxy(hydroxy)phosphoryl}oxy(hydroxy)phosphoryl)oxy]methyl}-2-hydroxy-3-methylbutanamido)propanamido]ethyl}sulfanyl)-2-hydroxy-4-oxobutanoic acid |
|---|
| SMILES | CC(C)(COP(O)(=O)OP(O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP(O)(O)=O)N1C=NC2=C1N=CN=C2N)C(O)C(=O)NCCC(=O)NCCSC(=O)C[C@H](O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C25H40N7O20P3S/c1-25(2,19(37)22(38)28-4-3-14(34)27-5-6-56-15(35)7-12(33)24(39)40)9-49-55(46,47)52-54(44,45)48-8-13-18(51-53(41,42)43)17(36)23(50-13)32-11-31-16-20(26)29-10-30-21(16)32/h10-13,17-19,23,33,36-37H,3-9H2,1-2H3,(H,27,34)(H,28,38)(H,39,40)(H,44,45)(H,46,47)(H2,26,29,30)(H2,41,42,43)/t12-,13+,17+,18+,19?,23+/m0/s1 |
|---|
| InChI Key | HJQWLHMLMCDAEL-NALABAGVSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as (r)-3-hydroxyacyl coas. These are organic compounds containing a (R)-3-hydroxyl acylated coenzyme A derivative. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty acyl thioesters |
|---|
| Direct Parent | (R)-3-hydroxyacyl CoAs |
|---|
| Alternative Parents | |
|---|
| Substituents | - Coenzyme a or derivatives
- Purine ribonucleoside 3',5'-bisphosphate
- Purine ribonucleoside bisphosphate
- Purine ribonucleoside diphosphate
- Pentose phosphate
- Pentose-5-phosphate
- Ribonucleoside 3'-phosphate
- Beta amino acid or derivatives
- Glycosyl compound
- N-glycosyl compound
- 6-aminopurine
- Monosaccharide phosphate
- Organic pyrophosphate
- Pentose monosaccharide
- Imidazopyrimidine
- Purine
- Aminopyrimidine
- Hydroxy fatty acid
- Monoalkyl phosphate
- Thia fatty acid
- Imidolactam
- Fatty amide
- Alpha-hydroxy acid
- Hydroxy acid
- Alkyl phosphate
- Monosaccharide
- N-acyl-amine
- N-substituted imidazole
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Pyrimidine
- Tetrahydrofuran
- Heteroaromatic compound
- Azole
- Imidazole
- Secondary carboxylic acid amide
- Secondary alcohol
- Carboxamide group
- Thiocarboxylic acid ester
- Amino acid
- Carbothioic s-ester
- Amino acid or derivatives
- Monocarboxylic acid or derivatives
- Sulfenyl compound
- Organoheterocyclic compound
- Thiocarboxylic acid or derivatives
- Azacycle
- Carboxylic acid derivative
- Oxacycle
- Carboxylic acid
- Organic oxygen compound
- Organic nitrogen compound
- Alcohol
- Organopnictogen compound
- Amine
- Carbonyl group
- Primary amine
- Organic oxide
- Hydrocarbon derivative
- Organonitrogen compound
- Organooxygen compound
- Organosulfur compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-1912000130-9228a9411607525f283a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0914000000-105c8023a5eeaf97f478 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-1911000000-6217731278e5194fdf7c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00lr-6911130370-1c5023b85b8d26542300 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-4910110010-1c24c172edfd42123b96 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-057i-5900100000-fdc66fff8f7a73dc20df | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00m0-0300000090-fa5f8e0b387a27651bfa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0901000050-d62b8d7e021a826dac17 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004i-0119000000-3a6de029d812bd3598fd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0000000090-726aaacba663c3277828 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-02ix-9000000740-f1f962d0fc0569a98ebb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-003r-7201304900-c7bf858de61078d27edb | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0001021 |
|---|
| FooDB ID | FDB022374 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | 5950 |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 389276 |
|---|
| ChEBI ID | 15454 |
|---|
| PubChem Compound ID | 440302 |
|---|
| Kegg Compound ID | C04348 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|