| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 05:25:30 UTC |
|---|
| Update Date | 2016-11-09 01:21:14 UTC |
|---|
| Accession Number | CHEM035021 |
|---|
| Identification |
|---|
| Common Name | 16-Dehydroprogesterone |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
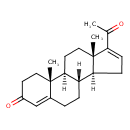
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3,20-Dioxopregna-4,16-diene | ChEBI | | Delta(4,16)-Pregnadiene-3,20-dione | ChEBI | | 16,17-Didehydroprogesterone | Kegg | | Δ(4,16)-pregnadiene-3,20-dione | Generator | | 4,16-Pregnadiene-3,20-dione | HMDB | | D16-Progesterone | HMDB | | D4,16-Pregnadiene-3,20-dione | HMDB | | Delta.16-progesterone | HMDB | | Delta4,16-Pregnadiene-3,20-dione | HMDB | | Pregna-4,16-diene-3,20-dione | HMDB | | delta(16)-Progesterone | HMDB | | 16-Dehydroprogesterone | ChEBI |
|
|---|
| Chemical Formula | C21H28O2 |
|---|
| Average Molecular Mass | 312.446 g/mol |
|---|
| Monoisotopic Mass | 312.209 g/mol |
|---|
| CAS Registry Number | 1096-38-4 |
|---|
| IUPAC Name | (1S,2R,10R,11S,15S)-14-acetyl-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-6,13-dien-5-one |
|---|
| Traditional Name | (1S,2R,10R,11S,15S)-14-acetyl-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-6,13-dien-5-one |
|---|
| SMILES | [H][C@@]12CC=C(C(C)=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2=CC(=O)CC[C@]12C |
|---|
| InChI Identifier | InChI=1S/C21H28O2/c1-13(22)17-6-7-18-16-5-4-14-12-15(23)8-10-20(14,2)19(16)9-11-21(17,18)3/h6,12,16,18-19H,4-5,7-11H2,1-3H3/t16-,18-,19-,20-,21+/m0/s1 |
|---|
| InChI Key | VRRHHTISESGZFN-RKFFNLMFSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 20-oxosteroids. These are steroid derivatives carrying a C=O group at the 20-position of the steroid skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Oxosteroids |
|---|
| Direct Parent | 20-oxosteroids |
|---|
| Alternative Parents | |
|---|
| Substituents | - 20-oxosteroid
- Androgen-skeleton
- Androstane-skeleton
- 3-oxosteroid
- Cyclohexenone
- Cyclic ketone
- Ketone
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-007k-0790000000-587e2010759d04be79da | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-03di-0009000000-70101cfe59fde51a8ff9 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-0a4j-5900000000-8c5e3f1358c9efe68931 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-0a4j-9500000000-1fa651a1bfd23c37c15b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0179000000-67a5fd6afc1237e6f0c5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01vt-0391000000-73037a7fa24c2f096328 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0f7a-2490000000-162c2aa3fd60d3279a3c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0019000000-f49571271662f7a5da14 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03xr-0098000000-4d72f69ebbb13d5f7c4e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0gbd-0090000000-4cd4d8acb44933c4d831 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0029000000-753e4d201cfb7cc7b807 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01ow-1493000000-67e624229aa746721027 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052f-7920000000-6f3d42fcb774cf571fa5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0009000000-152b659b4891e36b54f3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0049000000-4d4a6f7ba007f9c34c62 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-07fu-0192000000-c9494d220d3973cd4385 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0000995 |
|---|
| FooDB ID | FDB022357 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | 5926 |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 92118 |
|---|
| ChEBI ID | 18204 |
|---|
| PubChem Compound ID | 101964 |
|---|
| Kegg Compound ID | C03207 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|