| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 05:25:15 UTC |
|---|
| Update Date | 2016-11-09 01:21:14 UTC |
|---|
| Accession Number | CHEM035016 |
|---|
| Identification |
|---|
| Common Name | 4a-Methyl-5a-cholesta-8,24-dien-3-one |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
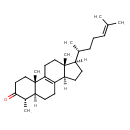
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (4alpha,5alpha)-4-Methylcholesta-8,24-dien-3-one | ChEBI | | 3-Keto-4alpha-methyl-zymosterol | ChEBI | | 3-Keto-4alpha-methylzymosterol | ChEBI | | 4alpha-Methyl-(5alpha)-cholesta-8,24-dien-3-one | ChEBI | | (4a,5a)-4-Methylcholesta-8,24-dien-3-one | Generator | | (4Α,5α)-4-methylcholesta-8,24-dien-3-one | Generator | | 3-Keto-4a-methyl-zymosterol | Generator | | 3-Keto-4α-methyl-zymosterol | Generator | | 3-Keto-4a-methylzymosterol | Generator | | 3-Keto-4α-methylzymosterol | Generator | | 4a-Methyl-(5a)-cholesta-8,24-dien-3-one | Generator | | 4Α-methyl-(5α)-cholesta-8,24-dien-3-one | Generator | | 4alpha-Methyl-5alpha-cholesta-8,24-dien-3-one | HMDB |
|
|---|
| Chemical Formula | C28H44O |
|---|
| Average Molecular Mass | 396.648 g/mol |
|---|
| Monoisotopic Mass | 396.339 g/mol |
|---|
| CAS Registry Number | 7377-73-3 |
|---|
| IUPAC Name | (2S,6S,7S,11R,14R,15R)-2,6,15-trimethyl-14-[(2R)-6-methylhept-5-en-2-yl]tetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadec-1(10)-en-5-one |
|---|
| Traditional Name | (2S,6S,7S,11R,14R,15R)-2,6,15-trimethyl-14-[(2R)-6-methylhept-5-en-2-yl]tetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadec-1(10)-en-5-one |
|---|
| SMILES | [H][C@@]1(CC[C@@]2([H])C3=C(CC[C@]12C)[C@@]1(C)CCC(=O)[C@@H](C)[C@]1([H])CC3)[C@H](C)CCC=C(C)C |
|---|
| InChI Identifier | InChI=1S/C28H44O/c1-18(2)8-7-9-19(3)22-12-13-24-21-10-11-23-20(4)26(29)15-17-28(23,6)25(21)14-16-27(22,24)5/h8,19-20,22-24H,7,9-17H2,1-6H3/t19-,20+,22-,23+,24+,27-,28+/m1/s1 |
|---|
| InChI Key | DBPZYKHQDWKORQ-SINUOACOSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as cholesterols and derivatives. Cholesterols and derivatives are compounds containing a 3-hydroxylated cholestane core. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Cholestane steroids |
|---|
| Direct Parent | Cholesterols and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Cholesterol-skeleton
- 3-oxo-5-alpha-steroid
- Oxosteroid
- 3-oxosteroid
- Cyclic ketone
- Ketone
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-02au-1019000000-74f38f1f24d48a20f4b0 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0009000000-1225e33fc8dbc85e29fd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0174-2009000000-71b5c36c965fb209ee16 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ap0-2119000000-e1899d14bd1d0142d0f1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0009000000-133fa32ff7ba267c45fd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0009000000-61f0749dd8a63d07401e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-02di-3009000000-3b2e5d918411006b6e76 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-002b-0009000000-dabf3142c40a4512bbdc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05p9-5589000000-dc34720a786d693f5d5e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-8942000000-801d53df4f599d0ae81b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0009000000-2f6aa9af0bd8878fb6c6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0009000000-2f6aa9af0bd8878fb6c6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-0009000000-ec12d6f6902d6552044d | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0000981 |
|---|
| FooDB ID | FDB022350 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | 5918 |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 20059529 |
|---|
| ChEBI ID | 136486 |
|---|
| PubChem Compound ID | 22298939 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|