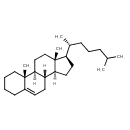| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 05:24:36 UTC |
|---|
| Update Date | 2016-11-09 01:21:13 UTC |
|---|
| Accession Number | CHEM035003 |
|---|
| Identification |
|---|
| Common Name | Cholest-5-ene |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 5-Cholestene | ChEBI | | D5-Cholestene | HMDB | | Cholest-5-ene | KEGG |
|
|---|
| Chemical Formula | C27H46 |
|---|
| Average Molecular Mass | 370.654 g/mol |
|---|
| Monoisotopic Mass | 370.360 g/mol |
|---|
| CAS Registry Number | 570-74-1 |
|---|
| IUPAC Name | (1S,2R,10S,11S,14R,15R)-2,15-dimethyl-14-[(2R)-6-methylheptan-2-yl]tetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadec-7-ene |
|---|
| Traditional Name | cholest-5-ene |
|---|
| SMILES | [H][C@@]12CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC=C2CCCC[C@]12C |
|---|
| InChI Identifier | InChI=1S/C27H46/c1-19(2)9-8-10-20(3)23-14-15-24-22-13-12-21-11-6-7-17-26(21,4)25(22)16-18-27(23,24)5/h12,19-20,22-25H,6-11,13-18H2,1-5H3/t20-,22+,23-,24+,25+,26+,27-/m1/s1 |
|---|
| InChI Key | DTGDZMYNKLTSKC-HKQCOZBKSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as cholestane steroids. These are steroids with a structure containing the 27-carbon cholestane skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Cholestane steroids |
|---|
| Direct Parent | Cholestane steroids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Cholestane-skeleton
- Delta-5-steroid
- Polycyclic hydrocarbon
- Cyclic olefin
- Unsaturated aliphatic hydrocarbon
- Unsaturated hydrocarbon
- Olefin
- Hydrocarbon
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4l-1229000000-e2078e492439a3aca82c | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-1119000000-64abbbde0fdcbb52f763 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05i0-5269000000-4cab5e10f9179e651209 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-8469000000-cb62741b93259b6ad9d8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0009000000-f8995c6171def5ad3530 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0009000000-6b9f58cc842b53f3a65a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udr-2139000000-74d55a222821b15f13ab | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0009000000-333124946ccb2f9590f8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05fr-9462000000-307fdd169d0e31c025a1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0aba-9430000000-fef5cdb755a2fa7fac79 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0009000000-e97c1bb5837bea46af05 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0009000000-e97c1bb5837bea46af05 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-0019000000-8fb2f12d17039ddc8e0b | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0000941 |
|---|
| FooDB ID | FDB022329 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00046564 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | 5889 |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 389543 |
|---|
| ChEBI ID | 28810 |
|---|
| PubChem Compound ID | 440663 |
|---|
| Kegg Compound ID | C05416 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Baker, Robert H.; Squire, Edward N. Derived steroids. I. Cholesteryl ketones. Journal of the American Chemical Society (1948), 70 1487-90. | | 2. Waters JA: Photosensitized isomerization-hydration of 5-cholestene. Steroids. 1974 Feb;23(2):259-67. | | 3. Swell L, Gustafsson J, Schwartz CC, Halloran LG, Danielsson H, Vlahcevic ZR: An in vivo evaluation of the quantitative significance of several potential pathways to cholic and chenodeoxycholic acids from cholesterol in man. J Lipid Res. 1980 May;21(4):455-66. | | 4. Bjorkhem I, Andersson U, Ellis E, Alvelius G, Ellegard L, Diczfalusy U, Sjovall J, Einarsson C: From brain to bile. Evidence that conjugation and omega-hydroxylation are important for elimination of 24S-hydroxycholesterol (cerebrosterol) in humans. J Biol Chem. 2001 Oct 5;276(40):37004-10. Epub 2001 Jul 19. | | 5. Esfahani M, Scerbo L, Devlin TM: A requirement for cholesterol and its structural features for a human macrophage-like cell line. J Cell Biochem. 1984;25(2):87-97. |
|
|---|