| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 05:22:46 UTC |
|---|
| Update Date | 2016-11-09 01:21:13 UTC |
|---|
| Accession Number | CHEM034967 |
|---|
| Identification |
|---|
| Common Name | Dimethylprotoporphyrin IX dimethyl ester |
|---|
| Class | Small Molecule |
|---|
| Description | The hepatic pigment accumulated as a consequence of the self-catalyzed destruction of cytochrome P-450 by norethisterone (PubMed ID 284396 ) [HMDB] |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
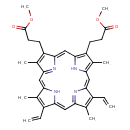
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Dimethyl 3,8,13,17-tetramethyl-7,12-divinyl-21H,23H-porphine-2,18-dipropionate | HMDB | | Dimethyl 7,12-diethenyl-3,8,13,17-tetramethyl-21H,23H-porphine-2,18-dipropanoate | HMDB | | Dimethyl 7,12-diethenyl-3,8,13,17-tetramethyl-21H,23H-porphine-2,18-dipropanoic acid | HMDB | | Dimethyl protoporphyrin IX | HMDB | | Protoporphyrin dimethyl ester | HMDB | | Protoporphyrin IX di-me ester | HMDB | | Protoporphyrin IX dimethyl ester | HMDB | | Methyl 3-[10,15-diethenyl-20-(3-methoxy-3-oxopropyl)-5,9,14,19-tetramethyl-21,22,23,24-tetraazapentacyclo[16.2.1.1³,⁶.1⁸,¹¹.1¹³,¹⁶]tetracosa-1(20),2,4,6(24),7,9,11,13(22),14,16,18-undecaen-4-yl]propanoic acid | HMDB |
|
|---|
| Chemical Formula | C36H38N4O4 |
|---|
| Average Molecular Mass | 590.711 g/mol |
|---|
| Monoisotopic Mass | 590.289 g/mol |
|---|
| CAS Registry Number | 5522-66-7 |
|---|
| IUPAC Name | methyl 3-[9,14-diethenyl-20-(3-methoxy-3-oxopropyl)-5,10,15,19-tetramethyl-21,22,23,24-tetraazapentacyclo[16.2.1.1^{3,6}.1^{8,11}.1^{13,16}]tetracosa-1,3,5,7,9,11(23),12,14,16,18(21),19-undecaen-4-yl]propanoate |
|---|
| Traditional Name | methyl 3-[9,14-diethenyl-20-(3-methoxy-3-oxopropyl)-5,10,15,19-tetramethyl-21,22,23,24-tetraazapentacyclo[16.2.1.1^{3,6}.1^{8,11}.1^{13,16}]tetracosa-1,3,5,7,9,11(23),12,14,16,18(21),19-undecaen-4-yl]propanoate |
|---|
| SMILES | COC(=O)CCC1=C2NC(\C=C3/N=C(/C=C4\N\C(=C/C5=N/C(=C\2)/C(CCC(=O)OC)=C5C)C(C)=C4C=C)C(C)=C3C=C)=C1C |
|---|
| InChI Identifier | InChI=1S/C36H38N4O4/c1-9-23-19(3)27-15-28-21(5)25(11-13-35(41)43-7)33(39-28)18-34-26(12-14-36(42)44-8)22(6)30(40-34)17-32-24(10-2)20(4)29(38-32)16-31(23)37-27/h9-10,15-18,37,40H,1-2,11-14H2,3-8H3/b27-15-,28-15-,29-16-,30-17-,31-16-,32-17-,33-18-,34-18- |
|---|
| InChI Key | WASRLAPXOHTNAX-MFBGAUBSSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as porphyrins. Porphyrins are compounds containing a fundamental skeleton of four pyrrole nuclei united through the alpha-positions by four methine groups to form a macrocyclic structure. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Tetrapyrroles and derivatives |
|---|
| Sub Class | Porphyrins |
|---|
| Direct Parent | Porphyrins |
|---|
| Alternative Parents | Not Available |
|---|
| Substituents | Not Available |
|---|
| Molecular Framework | Not Available |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-05o0-0000190000-a623429b2f83681d73d3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4l-0000090000-774674326ad0dac6319a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0apl-0000390000-4e2136815d15249f634e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052f-1000910000-9187995fb464c8ef2a70 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-052r-0000090000-160bde6cdb7c872647e8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0550-0000090000-32a69aea6125b72177f5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00bc-6000290000-92ad67b0d53ad4199eaf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-052f-0000090000-30528377d3ace2ba6ba5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0api-0000490000-4176d64c37e26119d9cd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0kmi-3000960000-6193640cb6937e68fce7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a6r-0000090000-35d4c1f7cf629b759126 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-056s-0000590000-a75fe7ac6a5603f40949 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00xr-0000930000-e95015955a0e465e95b7 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0000810 |
|---|
| FooDB ID | FDB022258 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | 5774 |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 10229562 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Jun, Ri Chol; Jin, Pak Song. Separation and purification of protoporphyrin IX. Choson Minjujuui Inmin Konghwaguk Kwahagwon Tongbo (1998), (6), 50-53. | | 2. Lee SH, Nam SY, Chung BC: Altered profile of endogenous steroids in the urine of patients with prolactinoma. Clin Biochem. 1998 Oct;31(7):529-35. | | 3. Nakajima O, Hashimoto Y, Iwasaki S: Erythroid differentiation-inducing activity of protoporphyrin IX and its analogs on human leukemia K562 cell line. Biochem Biophys Res Commun. 1994 Jan 28;198(2):720-7. | | 4. Pond AE, Roach MP, Sono M, Rux AH, Franzen S, Hu R, Thomas MR, Wilks A, Dou Y, Ikeda-Saito M, Ortiz de Montellano PR, Woodruff WH, Boxer SG, Dawson JH: Assignment of the heme axial ligand(s) for the ferric myoglobin (H93G) and heme oxygenase (H25A) cavity mutants as oxygen donors using magnetic circular dichroism. Biochemistry. 1999 Jun 8;38(23):7601-8. | | 5. Yee KK, Soo KC, Bay BH, Olivo M: A comparison of protoporphyrin IX and protoporphyrin IX dimethyl ester as a photosensitizer in poorly differentiated human nasopharyngeal carcinoma cells. Photochem Photobiol. 2002 Dec;76(6):678-82. |
|
|---|