| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 05:21:33 UTC |
|---|
| Update Date | 2016-11-09 01:21:13 UTC |
|---|
| Accession Number | CHEM034948 |
|---|
| Identification |
|---|
| Common Name | Hydroxypropionic porphyrin III |
|---|
| Class | Small Molecule |
|---|
| Description | Hydroxypropionic porphyrin III is a porphyrin isolated only in patients with porphyria cutanea tarda. Porphyria cutanea tarda (PCT) is a metabolic disorder of haeme biosynthesis caused by decreased activity of uroporphyrinogen decarboxylase. Porphyria cutanea tarda is manifest by fragility, erosions, bullae, milia and scars on sun-exposed skin. Excess porphyrins in the skin interact with light of approximately 400 nm-wavelength radiant energy, forming reactive oxygen species. Porphyria cutanea tarda is categorized as familial, acquired or toxic. Factors that may induce clinical expression of PCT in susceptible individuals include alcohol, oestrogen, iron, polyhalogenated compounds and viral infections. Porphyria cutanea tarda is associated with an increased incidence of the haemochromatosis gene. Treatments for PCT include withdrawal of aggravating factors, phlebotomy and oral antimalarial medications. Porphyria cutanea tarda is associated with hepatic iron overload and responds to iron-reduction therapy. Porphyria cutanea tarda is the most common porphyria, followed by acute intermittent porphyria and erythropoietic protoporphyria. The molecular genetics of the porphyrias is very heterogenous. (PMID: 16315139, 7655298, 11105361) [HMDB] |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
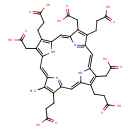
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| b-Hydroxypropionic acid heptacarboxylic porphyrin III | HMDB | | beta-Hydroxypropionic acid heptacarboxylic porphyrin III | HMDB | | 3-[10,15,20-Tris(2-carboxyethyl)-9,14,19-tris(carboxymethyl)-5-methyl-21,22,23,24-tetraazapentacyclo[16.2.1.1³,⁶.1⁸,¹¹.1¹³,¹⁶]tetracosa-1(20),2,4,6(24),7,9,11,13(22),14,16,18-undecaen-4-yl]propanoate | HMDB |
|
|---|
| Chemical Formula | C39H38N4O14 |
|---|
| Average Molecular Mass | 786.737 g/mol |
|---|
| Monoisotopic Mass | 786.238 g/mol |
|---|
| CAS Registry Number | 163894-03-9 |
|---|
| IUPAC Name | 3-[10,14,19-tris(2-carboxyethyl)-5,15,20-tris(carboxymethyl)-9-methyl-21,22,23,24-tetraazapentacyclo[16.2.1.1^{3,6}.1^{8,11}.1^{13,16}]tetracosa-1,3,5,7,9,11(23),12,14,16,18(21),19-undecaen-4-yl]propanoic acid |
|---|
| Traditional Name | 3-[10,14,19-tris(2-carboxyethyl)-5,15,20-tris(carboxymethyl)-9-methyl-21,22,23,24-tetraazapentacyclo[16.2.1.1^{3,6}.1^{8,11}.1^{13,16}]tetracosa-1,3,5,7,9,11(23),12,14,16,18(21),19-undecaen-4-yl]propanoic acid |
|---|
| SMILES | CC1=C(CCC(O)=O)C2=N\C\1=C/C1=C(CC(O)=O)C(CCC(O)=O)=C(N1)\C=C1/N=C(/C=C3\N/C(=C\2)C(CCC(O)=O)=C3CC(O)=O)C(CCC(O)=O)=C1CC(O)=O |
|---|
| InChI Identifier | InChI=1S/C39H38N4O14/c1-17-18(2-6-33(44)45)26-14-27-20(4-8-35(48)49)23(11-38(54)55)31(42-27)16-29-21(5-9-36(50)51)24(12-39(56)57)32(43-29)15-28-19(3-7-34(46)47)22(10-37(52)53)30(41-28)13-25(17)40-26/h13-16,41-42H,2-12H2,1H3,(H,44,45)(H,46,47)(H,48,49)(H,50,51)(H,52,53)(H,54,55)(H,56,57)/b25-13-,26-14-,27-14-,28-15-,29-16-,30-13-,31-16-,32-15- |
|---|
| InChI Key | NGHBDJAOMFYFRP-PKXAPQLQSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as porphyrins. Porphyrins are compounds containing a fundamental skeleton of four pyrrole nuclei united through the alpha-positions by four methine groups to form a macrocyclic structure. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Tetrapyrroles and derivatives |
|---|
| Sub Class | Porphyrins |
|---|
| Direct Parent | Porphyrins |
|---|
| Alternative Parents | Not Available |
|---|
| Substituents | Not Available |
|---|
| Molecular Framework | Not Available |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_4) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_5) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_6) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_7) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_8) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_9) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-106r-0000000900-5f02404e27194e023728 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05fs-0000002900-94a42c1c3c8c4e60e68a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-053s-0000009700-f3fa74f52ac07a4406b7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00xu-0000001900-17c885756ed1506e3c8b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0000002900-0b78b8f94f9aa3aaa643 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00di-2000000900-75b8d21b974b1fdd0063 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00dj-0000005900-5158682a2327ac61a264 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-006t-0000009600-376c90cfb6a9ee5df06b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00kb-0000009100-8b961aaf7f2ca7bd4150 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01dj-0000001900-cfa9a0533d6fc45d581b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0560-0000009500-b44d0a646f4c779ef9ef | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-003s-0000009500-1864e22e59aa72e2d360 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0000728 |
|---|
| FooDB ID | FDB022206 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | 5696 |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 17215996 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Luo, Jinli; Lim, Chang Kee. Isolation and characterization of new porphyrin metabolites in human porphyria cutanea tarda and in rats treated with hexachlorobenzene by HPTLC, HPLC and liquid secondary ion mass spectrometry. Biomedical Chromatography (1995), 9(3), 113-22. | | 2. Luo J, Lim CK: Isolation and characterization of new porphyrin metabolites in human porphyria cutanea tarda and in rats treated with hexachlorobenzene by HPTLC, HPLC and liquid secondary ion mass spectrometry. Biomed Chromatogr. 1995 May-Jun;9(3):113-22. | | 3. Hoffmann GF, Seppel CK, Holmes B, Mitchell L, Christen HJ, Hanefeld F, Rating D, Nyhan WL: Quantitative organic acid analysis in cerebrospinal fluid and plasma: reference values in a pediatric population. J Chromatogr. 1993 Jul 23;617(1):1-10. | | 4. Alla V, Bonkovsky HL: Iron in nonhemochromatotic liver disorders. Semin Liver Dis. 2005 Nov;25(4):461-72. | | 5. Bleasel NR, Varigos GA: Porphyria cutanea tarda. Australas J Dermatol. 2000 Nov;41(4):197-206; quiz 207-8. |
|
|---|