| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 05:17:29 UTC |
|---|
| Update Date | 2016-11-09 01:21:12 UTC |
|---|
| Accession Number | CHEM034870 |
|---|
| Identification |
|---|
| Common Name | 5a-Androstane-3a,17a-diol |
|---|
| Class | Small Molecule |
|---|
| Description | 5a-Androstane-3a,17a-diol is an steroid found in feces from pregnant women (PMID 5440628), primarily in the monosulfate and disulfate fractions from normal human (PMID 4245755), and glucuronide and mono- and disulphate conjugates of neutral steroids in human bile (PMID 5493463) [HMDB] |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
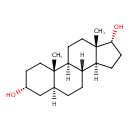
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 5a-Androstane-3a,17a-diol | Generator | | 5Α-androstane-3α,17α-diol | Generator | | 3a,17a-Dihydroxy-5a-androstane | HMDB | | (3alpha,5alpha,17alpha)-Androstane-3,17-diol | HMDB | | (3Α,5α,17α)-androstane-3,17-diol | HMDB | | 3alpha,17alpha-Dihydroxy-5alpha-androstane | HMDB | | 3Α,17α-dihydroxy-5α-androstane | HMDB | | 5alpha-Androstan-3alpha,17alpha-diol | HMDB | | 5Α-androstan-3α,17α-diol | HMDB | | 5alpha-Androstane-3alpha,17alpha-diol | HMDB |
|
|---|
| Chemical Formula | C19H32O2 |
|---|
| Average Molecular Mass | 292.456 g/mol |
|---|
| Monoisotopic Mass | 292.240 g/mol |
|---|
| CAS Registry Number | 6165-21-5 |
|---|
| IUPAC Name | (1S,2S,5R,7S,10R,11S,14R,15S)-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadecane-5,14-diol |
|---|
| Traditional Name | (1S,2S,5R,7S,10R,11S,14R,15S)-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadecane-5,14-diol |
|---|
| SMILES | [H][C@@]12CC[C@@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])C[C@H](O)CC[C@]12C |
|---|
| InChI Identifier | InChI=1S/C19H32O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h12-17,20-21H,3-11H2,1-2H3/t12-,13+,14-,15-,16-,17+,18-,19-/m0/s1 |
|---|
| InChI Key | CBMYJHIOYJEBSB-CUZKMJQKSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as androgens and derivatives. These are 3-hydroxylated C19 steroid hormones. They are known to favor the development of masculine characteristics. They also show profound effects on scalp and body hair in humans. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Androstane steroids |
|---|
| Direct Parent | Androgens and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Androgen-skeleton
- 17-hydroxysteroid
- 3-alpha-hydroxysteroid
- Hydroxysteroid
- 3-hydroxysteroid
- Cyclic alcohol
- Secondary alcohol
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Alcohol
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-03gi-0290000000-ef7ce05726cbec3a35bd | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-05fr-2227900000-87cd967afbbb2453594f | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004l-0090000000-ea970ea07cbd199d95ca | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-056r-0290000000-241c15d984d5502957c2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00mk-2790000000-1fb4fb943a8eb42cf30d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0090000000-7976b987ec10e61bdb4c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-006x-0090000000-72251dd83cca96a12d44 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01r5-0190000000-8a798c89a6c57c153169 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0090000000-11452f48ce72555f1f28 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-0090000000-11452f48ce72555f1f28 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000l-0090000000-ac9e93370094e8aebfa1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-002f-0090000000-36f4d97265b0d994d628 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052b-3940000000-b87bad8b672cc5fb32b0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0aos-3900000000-0f8ce10c286f165d6e0f | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0000458 |
|---|
| FooDB ID | FDB022056 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 8032954 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 9857254 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|