| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 05:10:35 UTC |
|---|
| Update Date | 2016-11-09 01:21:11 UTC |
|---|
| Accession Number | CHEM034741 |
|---|
| Identification |
|---|
| Common Name | N6-Carbamoyl-L-threonyladenosine |
|---|
| Class | Small Molecule |
|---|
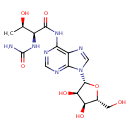
| Description | N6-Carbamoyl-L-threonyladenosine is a member of the class of compounds known as purine nucleosides. Purine nucleosides are compounds composed of a purine base attached to a ribosyl or deoxyribosyl moiety. N6-Carbamoyl-L-threonyladenosine is slightly soluble (in water) and a very weakly acidic compound (based on its pKa). Within the cell, N6-carbamoyl-L-threonyladenosine is primarily located in the cytoplasm. It can also be found in the extracellular space. N6-Carbamoyl-L-threonyladenosine is a minor constituent found in human and bovine milk (PMID: 7702711). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| N6-Carbamoylthreonyladenosine | HMDB | | (2S,3R)-N-{9-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-9H-purin-6-yl}-3-hydroxy-2-[(C-hydroxycarbonimidoyl)amino]butanimidate | HMDB |
|
|---|
| Chemical Formula | C15H20N6O8 |
|---|
| Average Molecular Mass | 412.355 g/mol |
|---|
| Monoisotopic Mass | 412.134 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | (2S,3R)-2-(carbamoylamino)-N-{9-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-9H-purin-6-yl}-3-hydroxybutanamide |
|---|
| Traditional Name | (2S,3R)-2-(carbamoylamino)-N-{9-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]purin-6-yl}-3-hydroxybutanamide |
|---|
| SMILES | C[C@@H](O)[C@H](NC(=O)NC1=NC=NC2=C1N=CN2[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C15H20N6O8/c1-5(23)7(14(26)27)19-15(28)20-11-8-12(17-3-16-11)21(4-18-8)13-10(25)9(24)6(2-22)29-13/h3-7,9-10,13,22-25H,2H2,1H3,(H,26,27)(H2,16,17,19,20,28)/t5-,6-,7+,9-,10-,13-/m1/s1 |
|---|
| InChI Key | UNUYMBPXEFMLNW-DWVDDHQFSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as purine nucleosides. Purine nucleosides are compounds comprising a purine base attached to a ribosyl or deoxyribosyl moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Nucleosides, nucleotides, and analogues |
|---|
| Class | Purine nucleosides |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Purine nucleosides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Purine nucleoside
- N-carbamoyl-alpha-amino acid or derivatives
- Alpha-amino acid amide
- Glycosyl compound
- N-glycosyl compound
- N-substituted-alpha-amino acid
- Alpha-amino acid or derivatives
- Pentose monosaccharide
- Imidazopyrimidine
- Purine
- N-arylamide
- Fatty amide
- Fatty acyl
- Monosaccharide
- N-substituted imidazole
- Pyrimidine
- Imidolactam
- Azole
- Tetrahydrofuran
- Imidazole
- Heteroaromatic compound
- Urea
- Secondary carboxylic acid amide
- Secondary alcohol
- Carbonic acid derivative
- Carboxamide group
- Organoheterocyclic compound
- Carboxylic acid derivative
- Azacycle
- Oxacycle
- Hydrocarbon derivative
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Organic nitrogen compound
- Carbonyl group
- Organonitrogen compound
- Organooxygen compound
- Alcohol
- Primary alcohol
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_4_50) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS ("N6-Carbamoyl-L-threonyladenosine,4TBDMS,#50" TMS) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03ec-0295100000-dd0a7f68fed335d54e87 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-0790000000-76fe4703eef1e5251d56 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-1940000000-f810a5bdef52a50d9a89 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00mo-7189200000-98389b90df31ce724bdb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-002g-4193000000-b93c6a0a2b4aeaaf5f6c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9450000000-2e740dcee3f05df70f94 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0059800000-898681b995fa652d206c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0192000000-f3fe7df861c514bcc4be | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03e9-2590000000-ff29117d97486cfd3ac0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03xr-0009400000-e1ea4bd3335c59980439 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01p5-2039000000-b1ed05fa3b95df27311e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03di-3960000000-79b7a9cecade3806a55c | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0041623 |
|---|
| FooDB ID | FDB021785 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 74854310 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 92021849 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Schlimme E, Schneehagen K: Ribonucleosides in human milk. Concentration profiles of these minor constituents as a function of the nursing time. Z Naturforsch C. 1995 Jan-Feb;50(1-2):105-13. | | 2. Elshenawy S, Pinney SE, Stuart T, Doulias PT, Zura G, Parry S, Elovitz MA, Bennett MJ, Bansal A, Strauss JF 3rd, Ischiropoulos H, Simmons RA: The Metabolomic Signature of the Placenta in Spontaneous Preterm Birth. Int J Mol Sci. 2020 Feb 4;21(3). pii: ijms21031043. doi: 10.3390/ijms21031043. |
|
|---|