| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 05:07:31 UTC |
|---|
| Update Date | 2016-11-09 01:21:10 UTC |
|---|
| Accession Number | CHEM034685 |
|---|
| Identification |
|---|
| Common Name | 2'-(4-Hydroxyphenylacetyl)-6'-(4-hydroxy-3-methylbutanoyl)-phloroacetophenone 4'-[rhamnosyl-(1->2)-glucoside] |
|---|
| Class | Small Molecule |
|---|
| Description | Not Available |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
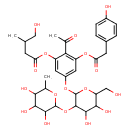
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-Acetyl-5-{[4,5-dihydroxy-6-(hydroxymethyl)-3-[(3,4,5-trihydroxy-6-methyloxan-2-yl)oxy]oxan-2-yl]oxy}-3-{[2-(4-hydroxyphenyl)acetyl]oxy}phenyl 4-hydroxy-3-methylbutanoic acid | Generator |
|
|---|
| Chemical Formula | C33H42O17 |
|---|
| Average Molecular Mass | 710.676 g/mol |
|---|
| Monoisotopic Mass | 710.242 g/mol |
|---|
| CAS Registry Number | 119558-02-0 |
|---|
| IUPAC Name | 2-acetyl-5-{[4,5-dihydroxy-6-(hydroxymethyl)-3-[(3,4,5-trihydroxy-6-methyloxan-2-yl)oxy]oxan-2-yl]oxy}-3-{[2-(4-hydroxyphenyl)acetyl]oxy}phenyl 4-hydroxy-3-methylbutanoate |
|---|
| Traditional Name | 2-acetyl-5-{[4,5-dihydroxy-6-(hydroxymethyl)-3-[(3,4,5-trihydroxy-6-methyloxan-2-yl)oxy]oxan-2-yl]oxy}-3-{[2-(4-hydroxyphenyl)acetyl]oxy}phenyl 4-hydroxy-3-methylbutanoate |
|---|
| SMILES | CC(CO)CC(=O)OC1=CC(OC2OC(CO)C(O)C(O)C2OC2OC(C)C(O)C(O)C2O)=CC(OC(=O)CC2=CC=C(O)C=C2)=C1C(C)=O |
|---|
| InChI Identifier | InChI=1S/C33H42O17/c1-14(12-34)8-23(38)47-20-10-19(11-21(25(20)15(2)36)48-24(39)9-17-4-6-18(37)7-5-17)46-33-31(29(43)27(41)22(13-35)49-33)50-32-30(44)28(42)26(40)16(3)45-32/h4-7,10-11,14,16,22,26-35,37,40-44H,8-9,12-13H2,1-3H3 |
|---|
| InChI Key | RODXFUFBAAPLPY-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as xanthophylls. These are carotenoids containing an oxygenated carotene backbone. Carotenes are characterized by the presence of two end-groups (mostly cyclohexene rings, but also cyclopentene rings or acyclic groups) linked by a long branched alkyl chain. Carotenes belonging form a subgroup of the carotenoids family. Xanthophylls arise by oxygenation of the carotene backbone. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Tetraterpenoids |
|---|
| Direct Parent | Xanthophylls |
|---|
| Alternative Parents | |
|---|
| Substituents | - Xanthophyll
- Fatty acid ester
- Fatty acyl
- Carboxylic acid ester
- Monocarboxylic acid or derivatives
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0f77-1613595100-d74f772c270f3e2e4f91 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4u-9312220000-12dc6b56b94542788392 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0w29-6944821000-980dcb9ce0de381f2102 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0r0s-4701496400-b1b557b27023f9c38eee | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0ika-1902352000-49afde9736e79a937096 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0w2a-7921600000-7abac9de47064e5e2391 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0101952000-0372facddc681bf0745f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-100a-0927560000-7095481701e3db7b71bb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4r-9711243000-1fe37d4000eb9c176107 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-052f-0301193100-12b336c48cf916b4685c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0904-2602793000-53a986f737832359b5a6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00kb-4192731000-669f832df250caaf060f | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0303777 |
|---|
| FooDB ID | FDB021649 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|