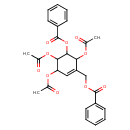| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 05:04:37 UTC |
|---|
| Update Date | 2016-11-09 01:21:09 UTC |
|---|
| Accession Number | CHEM034632 |
|---|
| Identification |
|---|
| Common Name | Piperenol A triacetate |
|---|
| Class | Small Molecule |
|---|
| Description | Piperenol A triacetate is found in herbs and spices. Piperenol A triacetate is a constituent of Piper cubeba (cubeb pepper). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Piperenol a triacetic acid | Generator | | [3,4,6-Tris(acetyloxy)-5-(benzoyloxy)cyclohex-1-en-1-yl]methyl benzoic acid | HMDB |
|
|---|
| Chemical Formula | C27H26O10 |
|---|
| Average Molecular Mass | 510.489 g/mol |
|---|
| Monoisotopic Mass | 510.153 g/mol |
|---|
| CAS Registry Number | 134476-92-9 |
|---|
| IUPAC Name | [3,4,6-tris(acetyloxy)-5-(benzoyloxy)cyclohex-1-en-1-yl]methyl benzoate |
|---|
| Traditional Name | [3,4,6-tris(acetyloxy)-5-(benzoyloxy)cyclohex-1-en-1-yl]methyl benzoate |
|---|
| SMILES | CC(=O)OC1C=C(COC(=O)C2=CC=CC=C2)C(OC(C)=O)C(OC(=O)C2=CC=CC=C2)C1OC(C)=O |
|---|
| InChI Identifier | InChI=1S/C27H26O10/c1-16(28)34-22-14-21(15-33-26(31)19-10-6-4-7-11-19)23(35-17(2)29)25(24(22)36-18(3)30)37-27(32)20-12-8-5-9-13-20/h4-14,22-25H,15H2,1-3H3 |
|---|
| InChI Key | UGMVPWAZGUPKPL-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pentacarboxylic acids and derivatives. These are carboxylic acids containing exactly five carboxyl groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Pentacarboxylic acids and derivatives |
|---|
| Direct Parent | Pentacarboxylic acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pentacarboxylic acid or derivatives
- Benzoate ester
- Benzoic acid or derivatives
- Benzoyl
- Benzenoid
- Monocyclic benzene moiety
- Cyclitol or derivatives
- Carboxylic acid ester
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4i-1916800000-fff82d9585598004d4be | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0n2i-0204920000-6bece60eb92b59cd59b4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a6r-0406900000-af11e59e5845a37fc27d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-2609400000-7ae8fb8b521a2cdb2f49 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-066r-3000940000-e009feb991f03a7ac29b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0axs-5403900000-cccaf58250d123accb6d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0ab9-9303200000-c535e25dc2ff6c8cfe71 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-06ri-0409550000-d59b7a2844fb35fd429e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-002b-0119000000-840ac849c2b463765d14 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4j-3948110000-ca557a6496e97c3bbd25 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-3209180000-7555b50855fdc2100aa8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0adi-9505600000-289b4c73fab9af013f07 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0560-7229200000-da16dcd6ccb4dbd19539 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0041535 |
|---|
| FooDB ID | FDB021515 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00057032 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35015198 |
|---|
| ChEBI ID | 172723 |
|---|
| PubChem Compound ID | 14890279 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|