| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 05:03:48 UTC |
|---|
| Update Date | 2016-11-09 01:21:09 UTC |
|---|
| Accession Number | CHEM034614 |
|---|
| Identification |
|---|
| Common Name | Glucopyranosyl anthranilate |
|---|
| Class | Small Molecule |
|---|
| Description | beta-D-Glucopyranosyl anthranilate is found in fruits. beta-D-Glucopyranosyl anthranilate is a constituent of the fruit of pi~nuela Bromelia plumieri. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
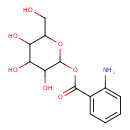
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| b-D-Glucopyranosyl anthranilate | Generator | | b-D-Glucopyranosyl anthranilic acid | Generator | | beta-D-Glucopyranosyl anthranilic acid | Generator | | Β-D-glucopyranosyl anthranilate | Generator | | Β-D-glucopyranosyl anthranilic acid | Generator | | 3,4,5-Trihydroxy-6-(hydroxymethyl)oxan-2-yl 2-aminobenzoic acid | Generator | | Glucopyranosyl anthranilic acid | Generator |
|
|---|
| Chemical Formula | C13H17NO7 |
|---|
| Average Molecular Mass | 299.277 g/mol |
|---|
| Monoisotopic Mass | 299.101 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl 2-aminobenzoate |
|---|
| Traditional Name | 3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl 2-aminobenzoate |
|---|
| SMILES | NC1=CC=CC=C1C(=O)OC1OC(CO)C(O)C(O)C1O |
|---|
| InChI Identifier | InChI=1S/C13H17NO7/c14-7-4-2-1-3-6(7)12(19)21-13-11(18)10(17)9(16)8(5-15)20-13/h1-4,8-11,13,15-18H,5,14H2 |
|---|
| InChI Key | GTQKOJVFDJDUGN-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as hexoses. These are monosaccharides in which the sugar unit is a is a six-carbon containing moeity. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Hexoses |
|---|
| Alternative Parents | |
|---|
| Substituents | - Hexose monosaccharide
- Aminobenzoic acid or derivatives
- Benzoate ester
- Benzoic acid or derivatives
- Benzoyl
- Aniline or substituted anilines
- Monocyclic benzene moiety
- Oxane
- Benzenoid
- Vinylogous amide
- Secondary alcohol
- Amino acid or derivatives
- Carboxylic acid ester
- Oxacycle
- Carboxylic acid derivative
- Polyol
- Organoheterocyclic compound
- Monocarboxylic acid or derivatives
- Acetal
- Primary amine
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Alcohol
- Amine
- Primary alcohol
- Organic nitrogen compound
- Organonitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0pir-9650000000-e110171444bec3734fce | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-00di-2911320000-194a8890697f50fbda88 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0080-0921000000-f532c1bf570386ae4b08 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-0900000000-0d410bb4a53ec16dd053 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00dr-6900000000-925548d4631874d820cd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00kv-2950000000-2cde06198b65478517ed | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000f-8910000000-a0d921265933e174c623 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9400000000-e67ac52d13b029025ba4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0fk9-0903000000-a49df83bb78846da1e53 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-3900000000-51405a736ca8414b1c5d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-006x-9510000000-148c624b804dab79e349 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-9310000000-5605ad3e1c91bdbebf4e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-9300000000-da0b651a85219e642d14 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9000000000-a56bb6561b73fe39863e | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0041517 |
|---|
| FooDB ID | FDB021492 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 75595895 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|