| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 05:00:34 UTC |
|---|
| Update Date | 2016-11-09 01:21:09 UTC |
|---|
| Accession Number | CHEM034557 |
|---|
| Identification |
|---|
| Common Name | Agrocybenine |
|---|
| Class | Small Molecule |
|---|
| Description | Agrocybenine is found in mushrooms. Agrocybenine is an alkaloid from the edible Korean mushroom yangimatusutake (Agrocybe cylindracea). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
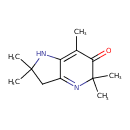
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1,2,3,5-tetrahydro-2,2,5,5,7-Pentamethyl-6H-pyrrolo[2,3-b]pyridin-6-one, 9ci | HMDB |
|
|---|
| Chemical Formula | C12H18N2O |
|---|
| Average Molecular Mass | 206.284 g/mol |
|---|
| Monoisotopic Mass | 206.142 g/mol |
|---|
| CAS Registry Number | 178764-92-6 |
|---|
| IUPAC Name | 2,2,5,5,7-pentamethyl-1H,2H,3H,5H,6H-pyrrolo[3,2-b]pyridin-6-one |
|---|
| Traditional Name | 2,2,5,5,7-pentamethyl-1H,3H-pyrrolo[3,2-b]pyridin-6-one |
|---|
| SMILES | CC1=C2NC(C)(C)CC2=NC(C)(C)C1=O |
|---|
| InChI Identifier | InChI=1S/C12H18N2O/c1-7-9-8(6-11(2,3)14-9)13-12(4,5)10(7)15/h14H,6H2,1-5H3 |
|---|
| InChI Key | OTBGQBSPIWKINO-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pyrrolopyridines. Pyrrolopyridines are compounds containing a pyrrolopyridine moiety, which consists of a pyrrole ring fused to a pyridine. Pyrrole is 5-membered ring consisting of four carbon atoms and one nitrogen atom. Pyridine is a 6-membered ring consisting of five carbon atoms and one nitrogen center. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pyrrolopyridines |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Pyrrolopyridines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyrrolopyridine
- Pyridine
- Pyrrolidine
- Vinylogous amide
- Ketimine
- Ketone
- Cyclic ketone
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Azacycle
- Secondary amine
- Secondary aliphatic amine
- Enamine
- Hydrocarbon derivative
- Organic nitrogen compound
- Organooxygen compound
- Organonitrogen compound
- Amine
- Imine
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Carbonyl group
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0udl-3900000000-004e49894baea0339d29 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0190000000-f3fe2bf3a97beeacc5dd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-1960000000-3e3b9550493822128da3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-9000000000-596872f3a3aa3a8fffe7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0090000000-b901ce8e24e79a027638 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-052b-9040000000-a5f283dd20be5a0335e5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03ki-2900000000-2dbc0df0e5f2d4fe189e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0090000000-f986a2b5346dd94f331a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0090000000-d01987e2e2a1ffcb3f4e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0fdp-5920000000-8f98f4b4afa2fc8fb5a8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0090000000-3e5ae49add9b8a734651 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-1970000000-daf84e5cdc2ad39ca18f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9500000000-e9bdb2b5e5d5db8fcd82 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0041445 |
|---|
| FooDB ID | FDB021396 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00027822 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 10446240 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 23278458 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|