| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 05:00:21 UTC |
|---|
| Update Date | 2016-11-09 01:21:09 UTC |
|---|
| Accession Number | CHEM034550 |
|---|
| Identification |
|---|
| Common Name | Pseudomonine |
|---|
| Class | Small Molecule |
|---|
| Description | Pseudomonine is found in fishes. Pseudomonine is an alkaloid from cultures of Pseudomonas fluorescens AH2 isolated from spoiled Nile perch from Lake Victoria. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
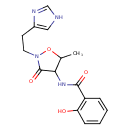
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-Hydroxy-N-{2-[2-(1H-imidazol-5-yl)ethyl]-5-methyl-3-oxo-1,2-oxazolidin-4-yl}benzene-1-carboximidate | HMDB | | Pseudomonine | MeSH |
|
|---|
| Chemical Formula | C16H18N4O4 |
|---|
| Average Molecular Mass | 330.339 g/mol |
|---|
| Monoisotopic Mass | 330.133 g/mol |
|---|
| CAS Registry Number | 172923-94-3 |
|---|
| IUPAC Name | 2-hydroxy-N-{2-[2-(1H-imidazol-4-yl)ethyl]-5-methyl-3-oxo-1,2-oxazolidin-4-yl}benzamide |
|---|
| Traditional Name | 2-hydroxy-N-{2-[2-(1H-imidazol-4-yl)ethyl]-5-methyl-3-oxo-1,2-oxazolidin-4-yl}benzamide |
|---|
| SMILES | CC1ON(CCC2=CNC=N2)C(=O)C1NC(=O)C1=C(O)C=CC=C1 |
|---|
| InChI Identifier | InChI=1S/C16H18N4O4/c1-10-14(19-15(22)12-4-2-3-5-13(12)21)16(23)20(24-10)7-6-11-8-17-9-18-11/h2-5,8-10,14,21H,6-7H2,1H3,(H,17,18)(H,19,22) |
|---|
| InChI Key | XYEWTJQWOJBDBL-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as n-acyl-alpha amino acids and derivatives. N-acyl-alpha amino acids and derivatives are compounds containing an alpha amino acid (or a derivative thereof) which bears an acyl group at its terminal nitrogen atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | N-acyl-alpha amino acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - N-acyl-alpha amino acid or derivatives
- Salicylamide
- Salicylic acid or derivatives
- Benzamide
- Benzoic acid or derivatives
- Benzoyl
- Phenol
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Monocyclic benzene moiety
- Oxazolidinone
- Benzenoid
- Heteroaromatic compound
- Azole
- Vinylogous acid
- Imidazole
- Isoxazolidine
- Carboxamide group
- Secondary carboxylic acid amide
- Organoheterocyclic compound
- Azacycle
- Oxacycle
- Organonitrogen compound
- Carbonyl group
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Organooxygen compound
- Organic nitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00di-9810000000-2afaeb864dbbccf9a295 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0006-9723000000-95363b07884822775aaf | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000t-9307000000-825490c91b72af8ddc63 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-2911000000-5df1831cc351f2211f47 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0f6t-9100000000-4fb6765e98ec90a6ffe7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0924000000-0231dc834b47ca7d6e10 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-6912000000-15892bf148d7bdc37550 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9500000000-e62a606994d2c0a9e58f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-1409000000-44f247efb610e8327bd0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-006x-9712000000-9696246936a231084222 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-9200000000-edc685c91d5e6271196f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-1619000000-fd28f0dab7a46b30955d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00ko-6962000000-51fc425c3ff1b2c1f462 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9210000000-2c5734b2390ae018c58e | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0041438 |
|---|
| FooDB ID | FDB021389 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00057144 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 154353 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 177258 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|