| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 04:59:34 UTC |
|---|
| Update Date | 2016-11-09 01:21:08 UTC |
|---|
| Accession Number | CHEM034533 |
|---|
| Identification |
|---|
| Common Name | Sarcodon scabrosus Depsipeptide |
|---|
| Class | Small Molecule |
|---|
| Description | Sarcodon scabrosus Depsipeptide is found in mushrooms. Sarcodon scabrosus Depsipeptide is isolated from the mushroom Sarcodon scabrosus of unknown palatability. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
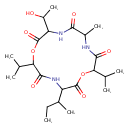
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C23H39N3O8 |
|---|
| Average Molecular Mass | 485.571 g/mol |
|---|
| Monoisotopic Mass | 485.274 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 3-(butan-2-yl)-9-(1-hydroxyethyl)-12-methyl-6,15-bis(propan-2-yl)-1,7-dioxa-4,10,13-triazacyclopentadecane-2,5,8,11,14-pentone |
|---|
| Traditional Name | 9-(1-hydroxyethyl)-6,15-diisopropyl-12-methyl-3-(sec-butyl)-1,7-dioxa-4,10,13-triazacyclopentadecane-2,5,8,11,14-pentone |
|---|
| SMILES | CCC(C)C1NC(=O)C(OC(=O)C(NC(=O)C(C)NC(=O)C(OC1=O)C(C)C)C(C)O)C(C)C |
|---|
| InChI Identifier | InChI=1S/C23H39N3O8/c1-9-12(6)15-22(31)33-17(10(2)3)20(29)24-13(7)19(28)26-16(14(8)27)23(32)34-18(11(4)5)21(30)25-15/h10-18,27H,9H2,1-8H3,(H,24,29)(H,25,30)(H,26,28) |
|---|
| InChI Key | ZZZCVVSDKFDQJU-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as cyclic depsipeptides. These are natural or synthetic compounds having sequences of amino and hydroxy carboxylic acid residues (usually α-amino and α-hydroxy acids) connected in a ring. The residues are commonly but not necessarily regularly alternating. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Peptidomimetics |
|---|
| Sub Class | Depsipeptides |
|---|
| Direct Parent | Cyclic depsipeptides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Cyclic depsipeptide
- Macrolide lactam
- Alpha-amino acid ester
- Macrolactam
- Macrolide
- Alpha-amino acid or derivatives
- Dicarboxylic acid or derivatives
- Carboxamide group
- Carboxylic acid ester
- Lactam
- Lactone
- Secondary carboxylic acid amide
- Secondary alcohol
- Carboxylic acid derivative
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Hydrocarbon derivative
- Organic oxide
- Alcohol
- Organopnictogen compound
- Carbonyl group
- Organic nitrogen compound
- Organic oxygen compound
- Organooxygen compound
- Organonitrogen compound
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-9000300000-755188e383f80122a750 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00mo-9200020000-83bf821dee0ef8987e9b | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0000900000-bc3a4ead6f8541f53fac | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-2000900000-e7d71f81a885aba58a9b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0zfr-4000900000-2c0b5d467442d9796fc4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0000900000-29eaf3f716447018d624 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-015c-0000900000-632084c9587b23397d43 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0avi-3002900000-b84c4508e0680605987f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0000900000-8067ba7a3aa1c9a9a01c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0000900000-8067ba7a3aa1c9a9a01c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0007-9000700000-147f1f98e5377fba007a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0000900000-c8c5cce128667921a7e0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-0000900000-c8c5cce128667921a7e0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-02i6-0002900000-fd55845c9846c729852e | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0041421 |
|---|
| FooDB ID | FDB021367 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35015180 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 131753140 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|