| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 04:57:07 UTC |
|---|
| Update Date | 2016-11-09 01:21:08 UTC |
|---|
| Accession Number | CHEM034486 |
|---|
| Identification |
|---|
| Common Name | Ustiloxin B |
|---|
| Class | Small Molecule |
|---|
| Description | Ustiloxin B is found in cereals and cereal products. Ustiloxin B is isolated from the false smut balls caused by Ustilaginoidea virens on rice. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
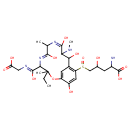
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-Amino-5-({4-[(carboxymethyl)-C-hydroxycarbonimidoyl]-3-ethyl-6,9,11,15-tetrahydroxy-3,7-dimethyl-10-(methylamino)-2-oxa-5,8-diazabicyclo[10.3.1]hexadeca-1(16),5,8,12,14-pentaen-13-yl}sulfinyl)-4-hydroxypentanoate | HMDB | | 2-Amino-5-({4-[(carboxymethyl)-C-hydroxycarbonimidoyl]-3-ethyl-6,9,11,15-tetrahydroxy-3,7-dimethyl-10-(methylamino)-2-oxa-5,8-diazabicyclo[10.3.1]hexadeca-1(16),5,8,12,14-pentaen-13-yl}sulphinyl)-4-hydroxypentanoate | HMDB | | 2-Amino-5-({4-[(carboxymethyl)-C-hydroxycarbonimidoyl]-3-ethyl-6,9,11,15-tetrahydroxy-3,7-dimethyl-10-(methylamino)-2-oxa-5,8-diazabicyclo[10.3.1]hexadeca-1(16),5,8,12,14-pentaen-13-yl}sulphinyl)-4-hydroxypentanoic acid | HMDB | | Ustiloxin b | MeSH |
|
|---|
| Chemical Formula | C26H39N5O12S |
|---|
| Average Molecular Mass | 645.679 g/mol |
|---|
| Monoisotopic Mass | 645.232 g/mol |
|---|
| CAS Registry Number | 151841-41-7 |
|---|
| IUPAC Name | 2-amino-5-{[(5Z,8E)-4-[(Z)-(carboxymethyl)-C-hydroxycarbonimidoyl]-3-ethyl-6,9,11,15-tetrahydroxy-3,7-dimethyl-10-(methylamino)-2-oxa-5,8-diazabicyclo[10.3.1]hexadeca-1(16),5,8,12,14-pentaen-13-yl]sulfinyl}-4-hydroxypentanoic acid |
|---|
| Traditional Name | 2-amino-5-[(5Z,8E)-4-[(Z)-carboxymethyl-C-hydroxycarbonimidoyl]-3-ethyl-6,9,11,15-tetrahydroxy-3,7-dimethyl-10-(methylamino)-2-oxa-5,8-diazabicyclo[10.3.1]hexadeca-1(16),5,8,12,14-pentaen-13-ylsulfinyl]-4-hydroxypentanoic acid |
|---|
| SMILES | CCC1(C)OC2=CC(C(O)C(NC)\C(O)=N/C(C)\C(O)=N\C1\C(O)=N\CC(O)=O)=C(C=C2O)S(=O)CC(O)CC(N)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C26H39N5O12S/c1-5-26(3)21(24(39)29-9-18(34)35)31-22(37)11(2)30-23(38)19(28-4)20(36)13-7-16(43-26)15(33)8-17(13)44(42)10-12(32)6-14(27)25(40)41/h7-8,11-12,14,19-21,28,32-33,36H,5-6,9-10,27H2,1-4H3,(H,29,39)(H,30,38)(H,31,37)(H,34,35)(H,40,41) |
|---|
| InChI Key | BISPUFPESHDUKH-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as diterpenoids. These are terpene compounds formed by four isoprene units. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Diterpenoids |
|---|
| Direct Parent | Diterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Diterpenoid
- Abietane diterpenoid
- Phenanthrene
- Hydrophenanthrene
- Tetralin
- Benzenoid
- Aromatic hydrocarbon
- Branched unsaturated hydrocarbon
- Polycyclic hydrocarbon
- Cyclic olefin
- Unsaturated hydrocarbon
- Olefin
- Hydrocarbon
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00bc-6000098000-869be0daa7793689910e | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_4) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_5) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_6) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_7) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_8) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_9) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_10) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_4) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_5) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_6) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_7) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_8) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_9) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_10) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_11) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_12) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_13) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_14) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00b9-4000039000-6a74fcd2af85d9ae6b70 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-9000010000-64f7eae1a2abff258e22 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-9000010000-f24527ae5c9e21a862b5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-01r6-1100169000-f2c0098daba031f06f1b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03dj-2100951000-ffed99fbdb28180e3237 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03di-8500910000-b6b4e94da144b9a59d13 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00dl-8200239000-d498e68c0e7e0cdb04f2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-022a-5000920000-8fd4ae039bf10ef6436e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03dl-9001610000-8057091590386846f403 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-006t-0200297000-d031c5188a3b237a893f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a6r-1100094000-a9ec60a9733fc1ae532e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03di-9600610000-898469021e0c3107089d | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0041373 |
|---|
| FooDB ID | FDB021299 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00016647 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35015164 |
|---|
| ChEBI ID | 167111 |
|---|
| PubChem Compound ID | 85067000 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|