| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 04:57:02 UTC |
|---|
| Update Date | 2016-11-09 01:21:08 UTC |
|---|
| Accession Number | CHEM034484 |
|---|
| Identification |
|---|
| Common Name | 18-Nor-4(19),8,11,13-abietatetraene |
|---|
| Class | Small Molecule |
|---|
| Description | 18-Nor-4(19),8,11,13-abietatetraene is isolated from oleoresin of Pinus koraiensis (Korean pine). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
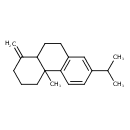
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 19-nordehydro-4(18)-Abietene | HMDB |
|
|---|
| Chemical Formula | C19H26 |
|---|
| Average Molecular Mass | 254.410 g/mol |
|---|
| Monoisotopic Mass | 254.203 g/mol |
|---|
| CAS Registry Number | 22478-62-2 |
|---|
| IUPAC Name | 4a-methyl-1-methylidene-7-(propan-2-yl)-1,2,3,4,4a,9,10,10a-octahydrophenanthrene |
|---|
| Traditional Name | 7-isopropyl-4a-methyl-1-methylidene-2,3,4,9,10,10a-hexahydrophenanthrene |
|---|
| SMILES | CC(C)C1=CC2=C(C=C1)C1(C)CCCC(=C)C1CC2 |
|---|
| InChI Identifier | InChI=1S/C19H26/c1-13(2)15-7-10-18-16(12-15)8-9-17-14(3)6-5-11-19(17,18)4/h7,10,12-13,17H,3,5-6,8-9,11H2,1-2,4H3 |
|---|
| InChI Key | ZPQHNIHJSIZREW-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as diterpenoids. These are terpene compounds formed by four isoprene units. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Diterpenoids |
|---|
| Direct Parent | Diterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Diterpenoid
- Abietane diterpenoid
- Phenanthrene
- Hydrophenanthrene
- Tetralin
- Benzenoid
- Aromatic hydrocarbon
- Branched unsaturated hydrocarbon
- Polycyclic hydrocarbon
- Cyclic olefin
- Unsaturated hydrocarbon
- Olefin
- Hydrocarbon
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-01ri-0290000000-6d5b5f7cad3bf99b06c2 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0090000000-fb11162c7750d2cbb7a4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0bt9-0790000000-8d65059303f41e438301 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01ri-4980000000-d760e192c1b922a9d668 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0090000000-cb44b94dc9c28b4d02ec | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0090000000-985b836f030f1472ab6f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000i-0190000000-f0f1d5dafb8539544d54 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0090000000-3dd9c0eb965ffcf9a18e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0bta-0970000000-3a2dcdfc5f4640648a73 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0536-5910000000-8666f04bfb3d57b0a92f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0090000000-238f16cf915f84d4c6b8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0090000000-238f16cf915f84d4c6b8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udr-0390000000-5b69979ed54c263871c5 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0041371 |
|---|
| FooDB ID | FDB021297 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00057263 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 4478352 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 5320205 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|