| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 04:55:54 UTC |
|---|
| Update Date | 2016-11-09 01:21:07 UTC |
|---|
| Accession Number | CHEM034457 |
|---|
| Identification |
|---|
| Common Name | Flupoxam |
|---|
| Class | Small Molecule |
|---|
| Description | Pre- and post emergence herbicide for broad-leaved weed control in winter cereals. |
|---|
| Contaminant Sources | - FooDB Chemicals
- STOFF IDENT Compounds
|
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-[4-chloro-3-[(2,2,3,3,3-Pentafluoropropoxy)methyl]phenyl]-5-phenyl-1H-1,2,4-triazole-3-carboxamide, 9ci | HMDB | | Flupoxam | HMDB | | KNW-739 | HMDB | | MON-18500 | HMDB |
|
|---|
| Chemical Formula | C19H14ClF5N4O2 |
|---|
| Average Molecular Mass | 460.785 g/mol |
|---|
| Monoisotopic Mass | 460.073 g/mol |
|---|
| CAS Registry Number | 119126-15-7 |
|---|
| IUPAC Name | 1-{4-chloro-3-[(2,2,3,3,3-pentafluoropropoxy)methyl]phenyl}-5-phenyl-1H-1,2,4-triazole-3-carboxamide |
|---|
| Traditional Name | flupoxam |
|---|
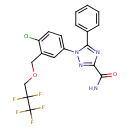
| SMILES | NC(=O)C1=NN(C(=N1)C1=CC=CC=C1)C1=CC=C(Cl)C(COCC(F)(F)C(F)(F)F)=C1 |
|---|
| InChI Identifier | InChI=1S/C19H14ClF5N4O2/c20-14-7-6-13(8-12(14)9-31-10-18(21,22)19(23,24)25)29-17(11-4-2-1-3-5-11)27-16(28-29)15(26)30/h1-8H,9-10H2,(H2,26,30) |
|---|
| InChI Key | AOQMRUTZEYVDIL-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenyl-1,2,4-triazoles. These are organic compounds containing a 1,2,4-triazole substituted by a phenyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Azoles |
|---|
| Sub Class | Triazoles |
|---|
| Direct Parent | Phenyl-1,2,4-triazoles |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenyl-1,2,4-triazole
- Benzylether
- 2-heteroaryl carboxamide
- Chlorobenzene
- Halobenzene
- Aryl chloride
- Aryl halide
- Monocyclic benzene moiety
- Benzenoid
- Heteroaromatic compound
- Carboxamide group
- Primary carboxylic acid amide
- Carboxylic acid derivative
- Azacycle
- Dialkyl ether
- Ether
- Organooxygen compound
- Organic nitrogen compound
- Alkyl halide
- Alkyl fluoride
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Organohalogen compound
- Organochloride
- Organofluoride
- Organonitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0wml-6639200000-d6df5580115c3a2c215b | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0603900000-e1b8a3f16d39cbad0216 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0519800000-810b7051a50fd04fb461 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-0901000000-88550633e11f21ba12ac | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-2200900000-0b7fd443e371ee581423 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4r-1400900000-666f171363fe422403c6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udi-4900000000-eb2e4bd4a92673284f48 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0001900000-681038bf1c11d4913e3a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001l-8091000000-4c02e5623c489e17cb53 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001l-9020000000-a16254d1449b1395e129 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0000900000-b5c408df863d79372aa8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0000900000-0f6a4b9fc9247a8d07fc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-0091000000-0377ae4e7e59d06cbd1c | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0041341 |
|---|
| FooDB ID | FDB021264 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 77871 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 86353 |
|---|
| Kegg Compound ID | C18543 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|