| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 04:54:57 UTC |
|---|
| Update Date | 2016-11-09 01:21:07 UTC |
|---|
| Accession Number | CHEM034436 |
|---|
| Identification |
|---|
| Common Name | Myristicanol A |
|---|
| Class | Small Molecule |
|---|
| Description | Myristicanol A is found in herbs and spices. Myristicanol A is a constituent of the arils of Myristica fragrans (mace). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
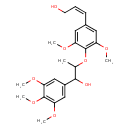
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| a-[1-[4-(3-Hydroxy-1-propenyl)-2,6-dimethoxyphenoxy]ethyl]-3,4,5-trimethoxybenzenemethanol, 9ci | HMDB |
|
|---|
| Chemical Formula | C23H30O8 |
|---|
| Average Molecular Mass | 434.480 g/mol |
|---|
| Monoisotopic Mass | 434.194 g/mol |
|---|
| CAS Registry Number | 114892-44-3 |
|---|
| IUPAC Name | (2Z)-3-(4-{[1-hydroxy-1-(3,4,5-trimethoxyphenyl)propan-2-yl]oxy}-3,5-dimethoxyphenyl)prop-2-en-1-ol |
|---|
| Traditional Name | (2Z)-3-(4-{[1-hydroxy-1-(3,4,5-trimethoxyphenyl)propan-2-yl]oxy}-3,5-dimethoxyphenyl)prop-2-en-1-ol |
|---|
| SMILES | COC1=CC(\C=C/CO)=CC(OC)=C1OC(C)C(O)C1=CC(OC)=C(OC)C(OC)=C1 |
|---|
| InChI Identifier | InChI=1S/C23H30O8/c1-14(21(25)16-12-19(28-4)22(30-6)20(13-16)29-5)31-23-17(26-2)10-15(8-7-9-24)11-18(23)27-3/h7-8,10-14,21,24-25H,9H2,1-6H3/b8-7- |
|---|
| InChI Key | QHYPOKHWZKVCEW-FPLPWBNLSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as lignans, neolignans and related compounds. These are plant products of low molecular weight formed primarily from oxidative coupling of two p-propylphenol moieties. They can also be described as micromolecules with two phenylpropanoid units coupled together. They can be attached in various manners, like C5-C5', C8-C8'. Most known natural lignans are oxidized at C9 and C9´ and, based upon the way in which oxygen is incorporated into the skeleton and on the cyclization patterns, a wide range of lignans of very different structural types can be formed. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lignans, neolignans and related compounds |
|---|
| Class | Not Available |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Lignans, neolignans and related compounds |
|---|
| Alternative Parents | |
|---|
| Substituents | - Neolignan skeleton
- Cinnamyl alcohol
- Dimethoxybenzene
- M-dimethoxybenzene
- Phenylpropane
- Methoxybenzene
- Phenoxy compound
- Phenol ether
- Anisole
- Styrene
- Alkyl aryl ether
- Monocyclic benzene moiety
- Benzenoid
- Secondary alcohol
- Ether
- Primary alcohol
- Organic oxygen compound
- Organooxygen compound
- Hydrocarbon derivative
- Alcohol
- Aromatic alcohol
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-014j-0930200000-93f8ff881d5cf188dc59 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-02ti-2190020000-0e66c552a46484027112 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00kr-0111900000-100f05bfe0646d1ba866 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-016r-1962300000-a7d9357529f7fa6cc203 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-016u-0930000000-e1780e2ac7c5602f22e7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0110900000-210e6040d1ec58a91c17 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-05nf-0950200000-e52a3afd5fa4d3934195 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-002f-0930000000-6f84e0617f7e417c82f5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0011900000-974d25ea2594b08ca516 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-002o-0925200000-26747996c41fa4976d45 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00kf-4911100000-d224805cc75d0061463d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014s-0033900000-7b9ee96e15c324122c7e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-0192200000-06618843b12a9b192afc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-016u-2942000000-2235e097284a7e3235f7 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0041311 |
|---|
| FooDB ID | FDB021230 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00024158 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35015151 |
|---|
| ChEBI ID | 176118 |
|---|
| PubChem Compound ID | 131753104 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|