| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 04:54:17 UTC |
|---|
| Update Date | 2016-11-09 01:21:07 UTC |
|---|
| Accession Number | CHEM034418 |
|---|
| Identification |
|---|
| Common Name | 6-Hydroxy-alpha-pyrufuran |
|---|
| Class | Small Molecule |
|---|
| Description | 6-Hydroxy-alpha-pyrufuran is found in fruits. 6-Hydroxy-alpha-pyrufuran is a constituent of the sapwood of Mespilus germanica (European medlar). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
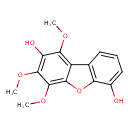
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 6-Hydroxy-a-pyrufuran | Generator | | 6-Hydroxy-α-pyrufuran | Generator | | 1,3,4-Trimethoxy-2,6-dibenzofurandiol | HMDB | | 2,6-Dihydroxy-1,3,4-trimethoxydibenzofuran | HMDB |
|
|---|
| Chemical Formula | C15H14O6 |
|---|
| Average Molecular Mass | 290.268 g/mol |
|---|
| Monoisotopic Mass | 290.079 g/mol |
|---|
| CAS Registry Number | 167278-43-5 |
|---|
| IUPAC Name | 3,5,6-trimethoxy-8-oxatricyclo[7.4.0.0²,⁷]trideca-1(9),2,4,6,10,12-hexaene-4,10-diol |
|---|
| Traditional Name | 3,5,6-trimethoxy-8-oxatricyclo[7.4.0.0²,⁷]trideca-1(9),2,4,6,10,12-hexaene-4,10-diol |
|---|
| SMILES | COC1=C2OC3=C(C=CC=C3O)C2=C(OC)C(O)=C1OC |
|---|
| InChI Identifier | InChI=1S/C15H14O6/c1-18-12-9-7-5-4-6-8(16)11(7)21-13(9)15(20-3)14(19-2)10(12)17/h4-6,16-17H,1-3H3 |
|---|
| InChI Key | XUGBHEYCYSYJSM-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dibenzofurans. Dibenzofurans are compounds containing a dibenzofuran moiety, which consists of two benzene rings fused to a central furan ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Benzofurans |
|---|
| Sub Class | Dibenzofurans |
|---|
| Direct Parent | Dibenzofurans |
|---|
| Alternative Parents | |
|---|
| Substituents | - Dibenzofuran
- Anisole
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Alkyl aryl ether
- Benzenoid
- Heteroaromatic compound
- Furan
- Oxacycle
- Ether
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-03di-0090000000-76d33de52ab4ed01cde0 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-01b9-3027900000-93cd274f54951541fb1e | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0090000000-5cbc9c09bfe1300afe3a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-0090000000-3bff7a84e83ab2e06a1b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0zos-1190000000-112c92e699e303f347c8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-7dd52c0c4e2e400ba740 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0079-0090000000-31ba2883fec95f05e054 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0uk9-2790000000-ac54b94ea8e5e980567b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0090000000-afe43bdc82c44950ba4a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-0090000000-afe43bdc82c44950ba4a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05r9-0790000000-c3247af320882444cf5b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-2d980c515d025d58a6fa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0090000000-50a978a1a3fea0a39b1d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udl-2290000000-4bb9ba334bdae3108a04 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0041293 |
|---|
| FooDB ID | FDB021210 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 8599722 |
|---|
| ChEBI ID | 174761 |
|---|
| PubChem Compound ID | 10424294 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|