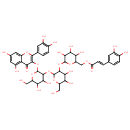| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 04:53:17 UTC |
|---|
| Update Date | 2016-11-09 01:21:07 UTC |
|---|
| Accession Number | CHEM034396 |
|---|
| Identification |
|---|
| Common Name | Quercetin 3-(6''-caffeoylsophorotrioside) |
|---|
| Class | Small Molecule |
|---|
| Description | Quercetin 3-(6''-caffeoylsophorotrioside) is found in pulses. Quercetin 3-(6''-caffeoylsophorotrioside) is a constituent of pea (Pisum sativum). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| [6-({2-[(2-{[2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-3-yl]oxy}-4,5-dihydroxy-6-(hydroxymethyl)oxan-3-yl)oxy]-4,5-dihydroxy-6-(hydroxymethyl)oxan-3-yl}oxy)-3,4,5-trihydroxyoxan-2-yl]methyl (2E)-3-(3,4-dihydroxyphenyl)prop-2-enoic acid | HMDB |
|
|---|
| Chemical Formula | C42H46O25 |
|---|
| Average Molecular Mass | 950.800 g/mol |
|---|
| Monoisotopic Mass | 950.233 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | [6-({2-[(2-{[2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-3-yl]oxy}-4,5-dihydroxy-6-(hydroxymethyl)oxan-3-yl)oxy]-4,5-dihydroxy-6-(hydroxymethyl)oxan-3-yl}oxy)-3,4,5-trihydroxyoxan-2-yl]methyl (2E)-3-(3,4-dihydroxyphenyl)prop-2-enoate |
|---|
| Traditional Name | [6-({2-[(2-{[2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxochromen-3-yl]oxy}-4,5-dihydroxy-6-(hydroxymethyl)oxan-3-yl)oxy]-4,5-dihydroxy-6-(hydroxymethyl)oxan-3-yl}oxy)-3,4,5-trihydroxyoxan-2-yl]methyl (2E)-3-(3,4-dihydroxyphenyl)prop-2-enoate |
|---|
| SMILES | OCC1OC(OC2C(O)C(O)C(CO)OC2OC2=C(OC3=CC(O)=CC(O)=C3C2=O)C2=CC(O)=C(O)C=C2)C(OC2OC(COC(=O)\C=C\C3=CC(O)=C(O)C=C3)C(O)C(O)C2O)C(O)C1O |
|---|
| InChI Identifier | InChI=1S/C42H46O25/c43-11-23-29(53)34(58)39(41(62-23)65-37-31(55)27-21(50)9-16(45)10-22(27)61-36(37)15-3-5-18(47)20(49)8-15)67-42-38(33(57)28(52)24(12-44)63-42)66-40-35(59)32(56)30(54)25(64-40)13-60-26(51)6-2-14-1-4-17(46)19(48)7-14/h1-10,23-25,28-30,32-35,38-50,52-54,56-59H,11-13H2/b6-2+ |
|---|
| InChI Key | XXCKFJNPDRDYOK-QHHAFSJGSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as flavonoid-3-o-glycosides. These are phenolic compounds containing a flavonoid moiety which is O-glycosidically linked to carbohydrate moiety at the C3-position. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Flavonoids |
|---|
| Sub Class | Flavonoid glycosides |
|---|
| Direct Parent | Flavonoid-3-O-glycosides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Oligosaccharide
- Flavonoid-3-o-glycoside
- Hydroxyflavonoid
- 3'-hydroxyflavonoid
- 4'-hydroxyflavonoid
- 5-hydroxyflavonoid
- 7-hydroxyflavonoid
- Flavone
- Hydroxycinnamic acid or derivatives
- Coumaric acid or derivatives
- Cinnamic acid ester
- Cinnamic acid or derivatives
- Chromone
- O-glycosyl compound
- Glycosyl compound
- Benzopyran
- 1-benzopyran
- Catechol
- Styrene
- Pyranone
- Fatty acid ester
- Phenol
- 1-hydroxy-2-unsubstituted benzenoid
- 1-hydroxy-4-unsubstituted benzenoid
- Monocyclic benzene moiety
- Oxane
- Fatty acyl
- Benzenoid
- Pyran
- Heteroaromatic compound
- Vinylogous acid
- Alpha,beta-unsaturated carboxylic ester
- Enoate ester
- Secondary alcohol
- Carboxylic acid ester
- Carboxylic acid derivative
- Organoheterocyclic compound
- Oxacycle
- Acetal
- Polyol
- Monocarboxylic acid or derivatives
- Organic oxygen compound
- Hydrocarbon derivative
- Organic oxide
- Carbonyl group
- Alcohol
- Primary alcohol
- Organooxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0529805104-29a4c944c33a64541101 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0439502000-dbf1598ab312d1d04179 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udi-0927201000-ae04b95839ea994568f4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0h01-0916423014-bdc874f0af2a251d4c10 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0in9-0916201002-190e55f11b3d7638f7ab | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0fb9-0926100000-44338a26f6c7d35f5096 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0000000009-5b1ffcc3516cd39e9942 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0f6t-0005000009-d337d7a7c60c3418c732 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udi-0019000001-62fdaf7471ee2969ac67 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0009000002-92ed4b0e3b03da48d612 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0009000009-9ff54c7419f1c425b374 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udi-0009000000-58b2754f892c40f07699 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0041266 |
|---|
| FooDB ID | FDB021177 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 131753092 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|