| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 04:52:27 UTC |
|---|
| Update Date | 2016-11-09 01:21:07 UTC |
|---|
| Accession Number | CHEM034378 |
|---|
| Identification |
|---|
| Common Name | Secoisotetrandrine |
|---|
| Class | Small Molecule |
|---|
| Description | Secoisotetrandrine is found in herbs and spices. Secoisotetrandrine is an alkaloid from leaves of Laurelia sempervirens (Peruvian nutmeg). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
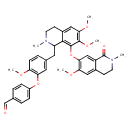
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C38H40N2O8 |
|---|
| Average Molecular Mass | 652.733 g/mol |
|---|
| Monoisotopic Mass | 652.278 g/mol |
|---|
| CAS Registry Number | 172924-26-4 |
|---|
| IUPAC Name | 4-[5-({6,7-dimethoxy-8-[(6-methoxy-2-methyl-1-oxo-1,2,3,4-tetrahydroisoquinolin-7-yl)oxy]-2-methyl-1,2,3,4-tetrahydroisoquinolin-1-yl}methyl)-2-methoxyphenoxy]benzaldehyde |
|---|
| Traditional Name | 4-[5-({6,7-dimethoxy-8-[(6-methoxy-2-methyl-1-oxo-3,4-dihydroisoquinolin-7-yl)oxy]-2-methyl-3,4-dihydro-1H-isoquinolin-1-yl}methyl)-2-methoxyphenoxy]benzaldehyde |
|---|
| SMILES | COC1=C(OC2=CC=C(C=O)C=C2)C=C(CC2N(C)CCC3=CC(OC)=C(OC)C(OC4=C(OC)C=C5CCN(C)C(=O)C5=C4)=C23)C=C1 |
|---|
| InChI Identifier | InChI=1S/C38H40N2O8/c1-39-15-14-26-20-34(45-5)36(46-6)37(48-33-21-28-25(19-31(33)44-4)13-16-40(2)38(28)42)35(26)29(39)17-24-9-12-30(43-3)32(18-24)47-27-10-7-23(22-41)8-11-27/h7-12,18-22,29H,13-17H2,1-6H3 |
|---|
| InChI Key | RMEYIAJCROYLDU-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as benzylisoquinolines. These are organic compounds containing an isoquinoline to which a benzyl group is attached. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Isoquinolines and derivatives |
|---|
| Sub Class | Benzylisoquinolines |
|---|
| Direct Parent | Benzylisoquinolines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Benzylisoquinoline
- Diphenylether
- Diaryl ether
- Isoquinolone
- Tetrahydroisoquinoline
- Anisole
- Phenoxy compound
- Benzaldehyde
- Benzoyl
- Methoxybenzene
- Phenol ether
- Alkyl aryl ether
- Aralkylamine
- Aryl-aldehyde
- Benzenoid
- Monocyclic benzene moiety
- Tertiary carboxylic acid amide
- Tertiary aliphatic amine
- Tertiary amine
- Amino acid or derivatives
- Carboxamide group
- Lactam
- Carboxylic acid derivative
- Ether
- Azacycle
- Hydrocarbon derivative
- Amine
- Organic oxide
- Organopnictogen compound
- Aldehyde
- Organic oxygen compound
- Organic nitrogen compound
- Organonitrogen compound
- Organooxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0100009000-baf0b78b94b1f91def0d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-11bc-0451229000-5fd75b348a7e55d82fe2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ab9-1921102000-a53a8df96b0d60f520ce | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0100009000-806ac9e0875795939f01 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0kmu-1310119000-6dc78d31530693d087d1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00di-1911200000-5b13bb24624e17372261 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0000019000-9bd901a655e7e73e0b6c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00fr-1100529000-debd838480897c8e6aeb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0nmj-1200249000-a47bc0f118fc70b8fecc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0000009000-a0fa0883b0dd99027c97 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0fkc-1200039000-92877a824921d7506504 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05fu-6301198000-415cf5ec1ac706ddcf9c | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0041246 |
|---|
| FooDB ID | FDB021154 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00057145 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35015138 |
|---|
| ChEBI ID | 169446 |
|---|
| PubChem Compound ID | 85174872 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|