| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 04:48:41 UTC |
|---|
| Update Date | 2016-11-09 01:21:06 UTC |
|---|
| Accession Number | CHEM034294 |
|---|
| Identification |
|---|
| Common Name | Cyanidin 3-O-[b-D-Xylopyranosyl-(1->2)-[(4-hydroxybenzoyl)-(->6)-b-D-glucopyranosyl-(1->6)]-b-D-galactopyranoside] |
|---|
| Class | Small Molecule |
|---|
| Description | Cyanidin 3-O-[b-D-Xylopyranosyl-(1->2)-[(4-hydroxybenzoyl)-(->6)-b-D-glucopyranosyl-(1->6)]-b-D-galactopyranoside] is found in root vegetables. Cyanidin 3-O-[b-D-Xylopyranosyl-(1->2)-[(4-hydroxybenzoyl)-(->6)-b-D-glucopyranosyl-(1->6)]-b-D-galactopyranoside] is isolated from carrot (Daucus carota). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
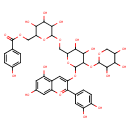
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3,3',4',5,7-Pentahydroxyflavylium(1+) | HMDB | | 3-O-[b-D-Xylopyranosyl-(1->2)-[(4-hydroxybenzoyl)-(->6)-b-D-glucopyranosyl-(1->6)]-b-D-galactopyranoside] | HMDB |
|
|---|
| Chemical Formula | C39H43O22 |
|---|
| Average Molecular Mass | 863.746 g/mol |
|---|
| Monoisotopic Mass | 863.225 g/mol |
|---|
| CAS Registry Number | 142506-20-5 |
|---|
| IUPAC Name | 3-({4,5-dihydroxy-6-[({3,4,5-trihydroxy-6-[(4-hydroxybenzoyloxy)methyl]oxan-2-yl}oxy)methyl]-3-[(3,4,5-trihydroxyoxan-2-yl)oxy]oxan-2-yl}oxy)-2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-1λ⁴-chromen-1-ylium |
|---|
| Traditional Name | 3-({4,5-dihydroxy-6-[({3,4,5-trihydroxy-6-[(4-hydroxybenzoyloxy)methyl]oxan-2-yl}oxy)methyl]-3-[(3,4,5-trihydroxyoxan-2-yl)oxy]oxan-2-yl}oxy)-2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-1λ⁴-chromen-1-ylium |
|---|
| SMILES | OC1COC(OC2C(O)C(O)C(COC3OC(COC(=O)C4=CC=C(O)C=C4)C(O)C(O)C3O)OC2OC2=CC3=C(O)C=C(O)C=C3[O+]=C2C2=CC=C(O)C(O)=C2)C(O)C1O |
|---|
| InChI Identifier | InChI=1S/C39H42O22/c40-16-4-1-14(2-5-16)36(53)54-12-25-28(47)30(49)33(52)37(59-25)56-13-26-29(48)31(50)35(61-38-32(51)27(46)22(45)11-55-38)39(60-26)58-24-10-18-20(43)8-17(41)9-23(18)57-34(24)15-3-6-19(42)21(44)7-15/h1-10,22,25-33,35,37-39,45-52H,11-13H2,(H4-,40,41,42,43,44,53)/p+1 |
|---|
| InChI Key | NKWMVPVTAGESHZ-UHFFFAOYSA-O |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as flavonoid-3-o-glycosides. These are phenolic compounds containing a flavonoid moiety which is O-glycosidically linked to carbohydrate moiety at the C3-position. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Flavonoids |
|---|
| Sub Class | Flavonoid glycosides |
|---|
| Direct Parent | Flavonoid-3-O-glycosides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Oligosaccharide
- Flavonoid-3-o-glycoside
- 3'-hydroxyflavonoid
- 4'-hydroxyflavonoid
- 7-hydroxyflavonoid
- Hydroxyflavonoid
- O-glycosyl compound
- Glycosyl compound
- Benzopyran
- 1-benzopyran
- Benzoic acid or derivatives
- Benzoic acid
- Catechol
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Oxane
- Benzenoid
- Monocyclic benzene moiety
- Heteroaromatic compound
- Vinylogous acid
- Secondary alcohol
- Polyol
- Carboxylic acid derivative
- Carboximidic acid
- Organoheterocyclic compound
- Oxacycle
- Acetal
- Monocarboxylic acid or derivatives
- Organic oxide
- Organic oxygen compound
- Alcohol
- Hydrocarbon derivative
- Organooxygen compound
- Organic cation
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0190000310-f88a6e334d4ed5cb9d99 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0190100000-8f97b7b8296aa09bb05e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0079-0590100000-fff02e0e74f4c5e44882 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001r-0140130940-55954064576f857e65d5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03ei-0960860350-b5aa0080d267f80978e9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00y0-1960230010-edfb32ed4e7652b4600d | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0041160 |
|---|
| FooDB ID | FDB021049 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 74976921 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|