| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 04:47:59 UTC |
|---|
| Update Date | 2016-11-09 01:21:06 UTC |
|---|
| Accession Number | CHEM034277 |
|---|
| Identification |
|---|
| Common Name | 8-Methyldihydrochelerythrine |
|---|
| Class | Small Molecule |
|---|
| Description | Alkaloid from root bark of Zanthoxylum simulans (Szechuan pepper). 8-Methyldihydrochelerythrine is found in herbs and spices and fruits. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
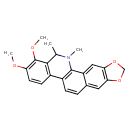
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 6-Methyldihydro-chelerythrine | HMDB | | 6-Methyldihydrochelerythrine | MeSH |
|
|---|
| Chemical Formula | C22H21NO4 |
|---|
| Average Molecular Mass | 363.406 g/mol |
|---|
| Monoisotopic Mass | 363.147 g/mol |
|---|
| CAS Registry Number | 159465-79-9 |
|---|
| IUPAC Name | 17,18-dimethoxy-20,21-dimethyl-5,7-dioxa-21-azapentacyclo[11.8.0.0²,¹⁰.0⁴,⁸.0¹⁴,¹⁹]henicosa-1(13),2,4(8),9,11,14(19),15,17-octaene |
|---|
| Traditional Name | 17,18-dimethoxy-20,21-dimethyl-5,7-dioxa-21-azapentacyclo[11.8.0.0²,¹⁰.0⁴,⁸.0¹⁴,¹⁹]henicosa-1(13),2,4(8),9,11,14(19),15,17-octaene |
|---|
| SMILES | COC1=C(OC)C2=C(C=C1)C1=C(N(C)C2C)C2=CC3=C(OCO3)C=C2C=C1 |
|---|
| InChI Identifier | InChI=1S/C22H21NO4/c1-12-20-14(7-8-17(24-3)22(20)25-4)15-6-5-13-9-18-19(27-11-26-18)10-16(13)21(15)23(12)2/h5-10,12H,11H2,1-4H3 |
|---|
| InChI Key | NDRJOHZGHCUTCQ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dihydrobenzophenanthridine alkaloids. These are alkaloids containing a dihydrobenzophenanthridine skeleton, which is a tetracyclic compound containing a benzene fused to a dihydrophenanthridine moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Alkaloids and derivatives |
|---|
| Class | Benzophenanthridine alkaloids |
|---|
| Sub Class | Dihydrobenzophenanthridine alkaloids |
|---|
| Direct Parent | Dihydrobenzophenanthridine alkaloids |
|---|
| Alternative Parents | Not Available |
|---|
| Substituents | Not Available |
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0002-0019000000-2f48c6b86ae786f3062a | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0009000000-295c0f5f691a94da1f66 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03e9-0019000000-94943f2615fcc443114f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0l6r-0149000000-43c15cb28cb4e12044fb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0009000000-fb873dbe74b71b902c82 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0009000000-bda573b667eb0bd09cb7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014u-0094000000-98374669634722f55d7f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0009000000-6fc89c82ef07a22611d6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0009000000-4382821ef8a46bfb119c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03yi-0019000000-aa69aa9ac8aa4584ad64 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0009000000-e26737f0e2e36e925666 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0009000000-74074607c6d61e73b881 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00lr-0019000000-fc8bbba07ec62e2689de | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0041143 |
|---|
| FooDB ID | FDB021029 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 101673184 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|